-
放射治疗(简称放疗)在治疗胸部恶性肿瘤(包括食道癌、乳腺癌和非小细胞肺癌)中起着重要作用[1]。为了尽量减少放疗后放射性肺纤维化(radioactive pulmonary fibrosis,RIPF)的风险,行放疗时的辐射剂量通常受到限制。放射性肺损伤的表现包括急性放射性肺炎和慢性RIPF。虽然放疗技术的进步显著降低了不良反应,但仍有近15%的胸部放疗患者出现放射性肺炎和慢性RIPF[2]。
诱导RIPF形成的方式随照射仪器、射线种类、照射方式以及物种的不同而差异显著[3]。例如,在C57BL/6小鼠模型中,行胸部照射6个月后(剂量约为15 Gy),小鼠会出现明显的RIPF[4]。采用20 Gy照射2个月龄SD大鼠的右肺,发现受照射的肺体积萎缩、质地硬韧,6个月后出现对侧肺代偿性肥大。雄性SD大鼠单侧胸部照射20 Gy后12周,I型和II型上皮细胞和内皮细胞数量减少了50%~70%,同时毛细血管内血液总量减少了75%,间质细胞的数量增加了3倍。照射后26周,肺间质中肥大细胞数目增长了540倍,其他渗出液和浆细胞数目增长了180倍[5-6]。
相对于小鼠的RIPF模型,大鼠肺部体积更大,更适于RIPF的功能研究,而且从进化上来说,大鼠要早于小鼠[7]。因此,本研究拟采用X射线辐照仪对SD大鼠行单侧右肺照射,以建立SD大鼠RIPF模型,并探寻实验中纤维化蛋白、细胞因子等作为组织纤维化程度评价指标的可行性,为RIPF的研究提供基础。
HTML
-
放射免疫沉淀法(radio immunoprecipitation assay,RIPA)裂解液购自北京索莱宝公司;BCA蛋白浓度测定试剂盒购自上海碧云天公司;纤维黏连蛋白1(fibronectin1,FN1)、基质金属蛋白酶2(matrix metalloproteinase 2,MMP2)、α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)抗体购自英国Abcam公司;小鼠抗人甘油醛-3-磷酸脱氢酶、山羊抗鼠(兔)免疫球蛋白G抗体(辣根过氧化物酶标记)购自美国Proteintech公司;普通电化学发光检测试剂盒购自美国Proteintech公司;羟脯氨酸(hydroxyproline,HYP)含量检测试剂盒购自北京索莱宝公司;酶联免疫吸附测定(enzyme-linked immunosorbent assay,ELISA)试剂盒购自美国R&D公司;生物学X射线辐照仪RS-2000购自美国RAD SOURCE公司;多功能酶标仪购自美国Bio Tek公司;超滤离心管购自美国Millipore公司;离心机Centrifuge 5424 R购自德国Eppendorf公司。
-
SD雄性大鼠购自北京华阜康生物科技股份有限公司,实验动物许可证号:SCXK(京)2019-0008。经中国医学科学院医学实验动物研究所质量检测后,将周龄4~6周、体重为180~200 g的37只SD雄性大鼠置于无特定病原体级饲养环境中。将实验动物完全随机分为4组:对照组(5只)、13 Gy照射组(10只)、15 Gy照射组(10只)和17 Gy照射组(12只),行X射线单侧右肺照射,剂量率为1.522 Gy/min。分别于照后4个月和6个月处死并取材。所有实验都在动物麻醉下无痛进行。
-
取右肺第二叶肺标本,经4%甲醛溶液固定,石蜡包埋,5 mm切片。肺切片用苏木精-伊红(hematoxylin-eosin,HE)染色和马松三色染色,操作步骤按说明书进行。选取10个随机、不重叠的肺实质视野(10倍、20倍放大)观察和记录RIPF。
-
支气管肺泡灌洗采用气管插管,结扎左肺和右肺第二叶,进行其余肺叶段灌洗,用生理盐水反复冲洗3次,收集肺泡支气管灌洗液(bronchoalveolar lavage fluid,BALF)。
-
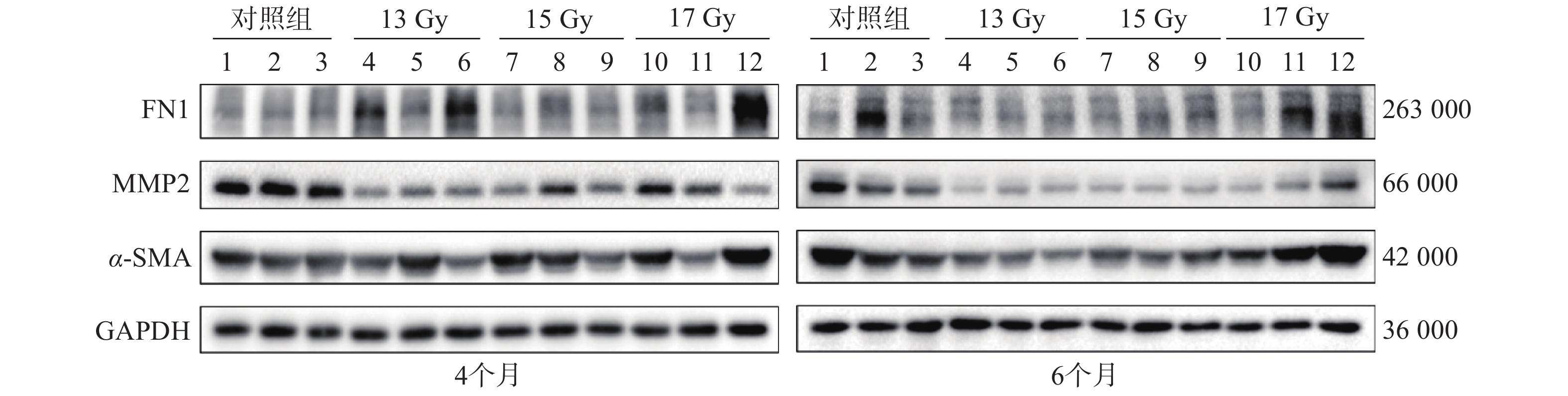
选取各组中的部分肺组织样本,采用Western blot检测基质蛋白FN1、MMP2、α-SMA的表达。肺组织在RIPA裂解液(150 mmol/L NaCl、50 mmol/L Tris、pH=8.0、1% Triton X-100、0.5%脱氧胆酸钠、0.1%十二烷基硫酸钠、外加蛋白酶和磷酸酶抑制剂)中裂解完全后,用BCA蛋白浓度测定试剂盒进行蛋白浓度定量。采用十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离溴酚蓝缓冲液中等量的蛋白质,转移到聚偏二氟乙烯膜中,用1%牛血清白蛋白 BSA-Ⅴ封闭,然后用稀释后的FN1、MMP2、α-SMA抗体孵育,再用相应的生物素二抗孵育,最后使用普通电化学发光检测试剂盒在凝胶成像仪中检测蛋白信号。
-
HYP是胶原纤维的主要成分之一,因此可通过检测肺组织HYP的含量来评估肺纤维化严重程度。称取约0.2 g肺组织,剪碎后加入2 mL的提取液,煮沸2~6 h消化完全后,离心20 min(离心力为20 000 g),用10 mol/L NaOH调节pH值至6~8。蒸馏水定容至4 mL,取上清,按测定操作表检测波长560 nm处的吸光度值,计算肺组织的HYP含量。
-
收集大鼠右肺的BALF,接着使用超滤离心管在4℃下5000 g离心30 min,浓缩蛋白,按照ELISA试剂盒说明书,定量检测转化生长因子β(transforming growth factor β,TGF-β)、白细胞介素6(interleukin 1,IL-6)、TNF-α、γ干扰素(interferon γ,INF-γ)的含量。
-
采用IBM SPSS Statistics 22软件进行统计学分析。符合正态分布的计量资料以均数±标准差(
${\bar {x}} \pm {s}$
1.1. 试剂与仪器
1.2. 实验动物及分组
1.3. 免疫组化
1.4. 支气管肺泡灌洗
1.5. Western blot检测
1.6. HYP含量测定
1.7. ELISA方法
1.8. 统计学方法
-
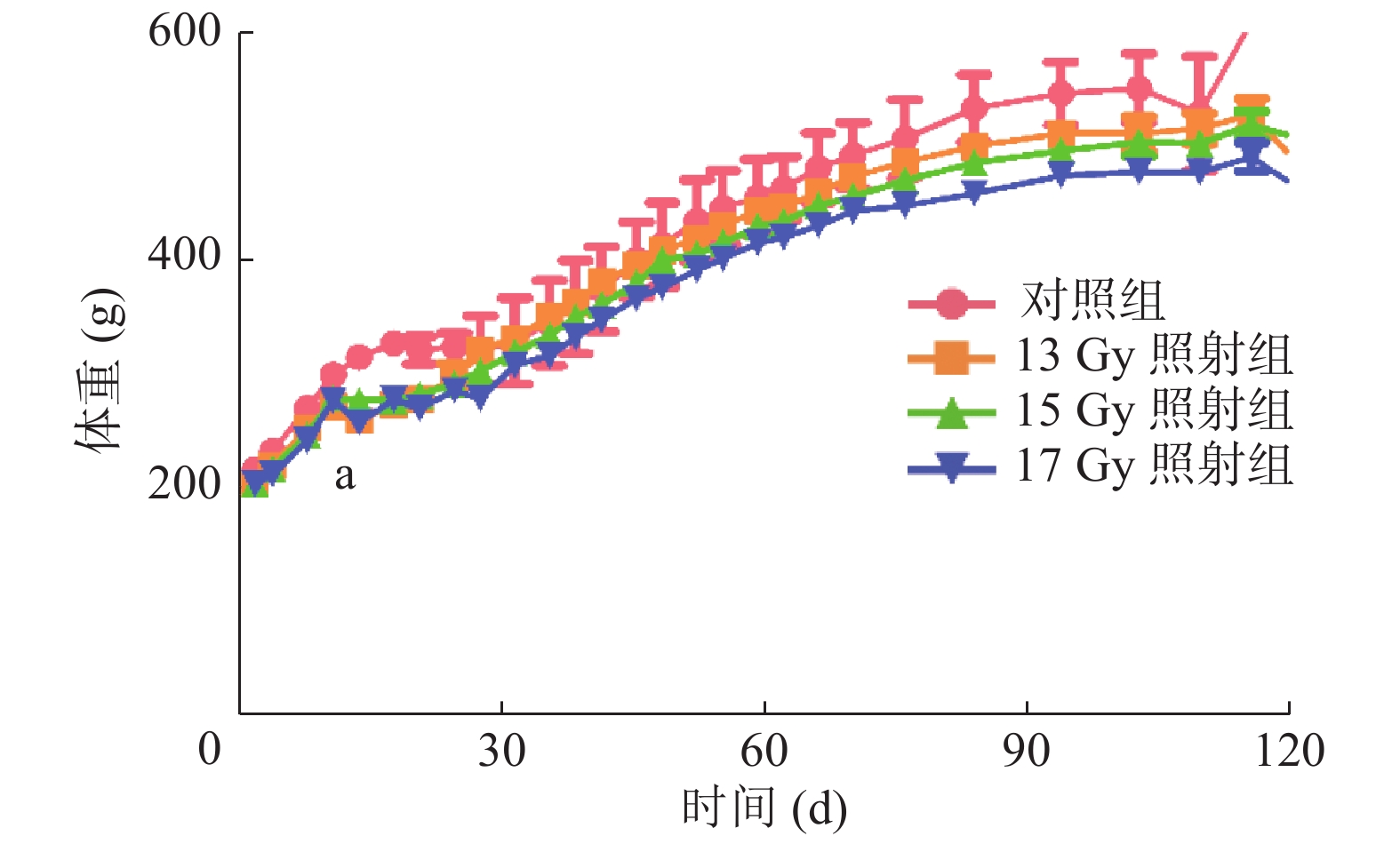
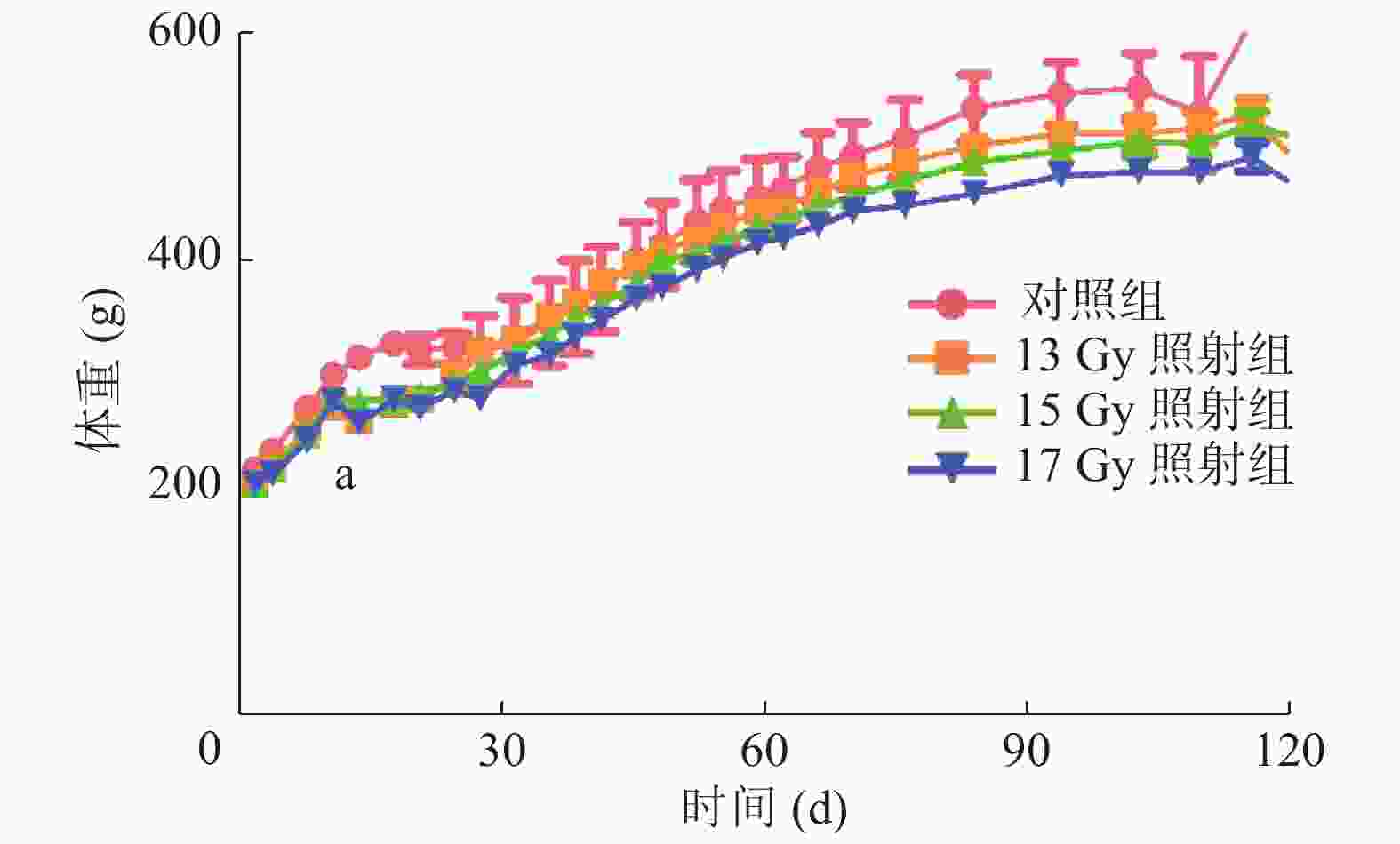
观察记录SD大鼠6个月的体重变化,与对照组相比,照射组SD大鼠体重均不同程度地下降,尤其在照射后第13天,体重明显下降,对照组为(317.47 ±9.07)g,13、15、17 Gy照射组分别为(261.69 ±22.16)、(279.32 ±17.81)、(259.66 ±10.54)g,差异均有统计学意义(t=4.151、3.499、8.672,均P<0.05)(图1)。
-
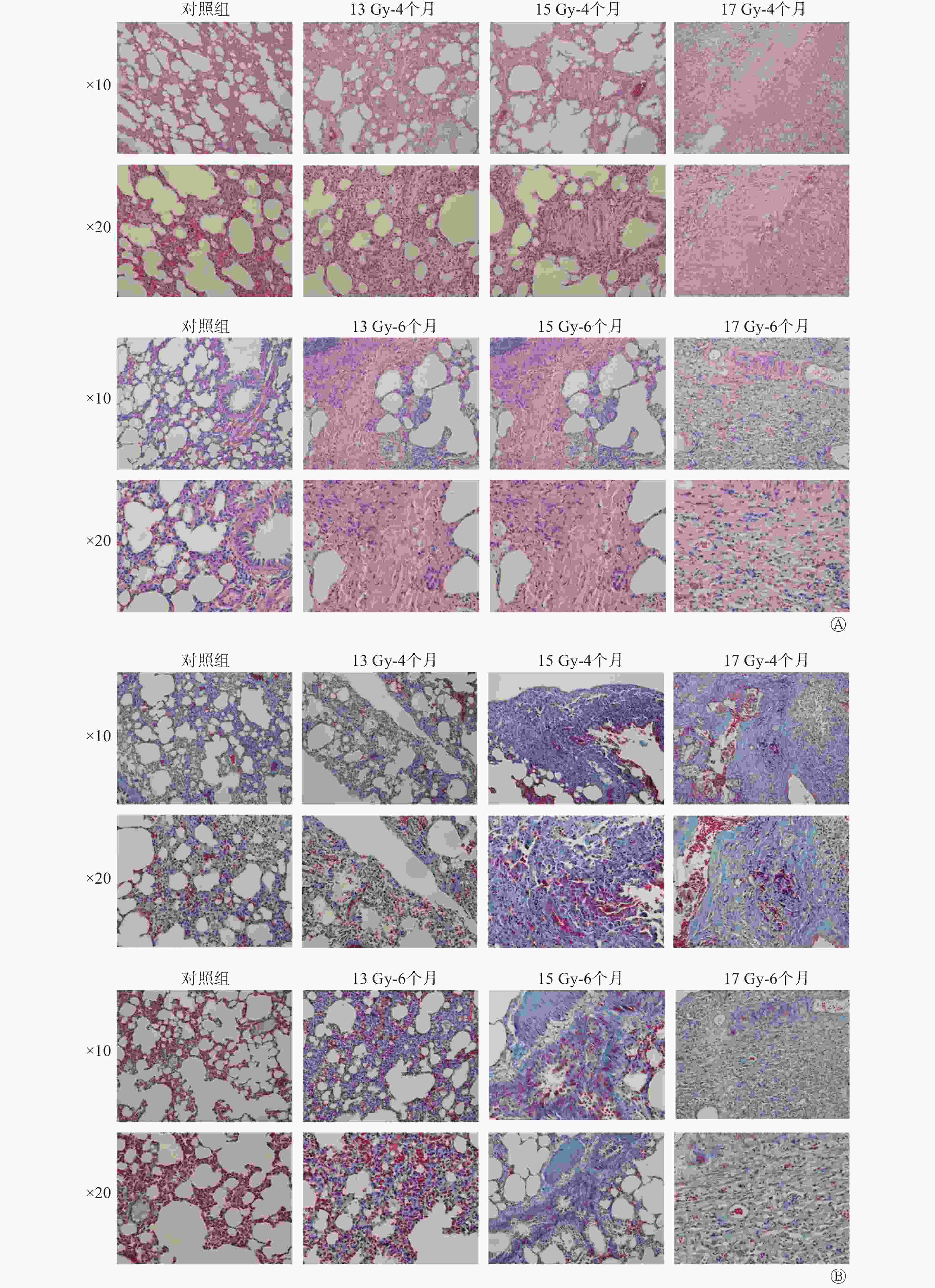
HE染色结果显示(图2中A),照射后4个月,不同剂量组均显示间质细胞大量增加,肺泡壁间隔增宽,肺泡的完整性降低,肺质地较对照组稍增厚。马松三色染色结果显示(图2中B),照射4个月后,肺组织显示不同程度的胶原纤维沉积(蓝色),6个月后局部区域的纤维化更为明显,且随着剂量的增加纤维化更为严重,提示13、15、17 Gy均可诱导大鼠RIPF模型的建立。
-
Western blot检测结果显示,与对照组相比,照射组样本中FN1和α-SMA蛋白表达增加,MMP2蛋白表达降低(图3)。
-
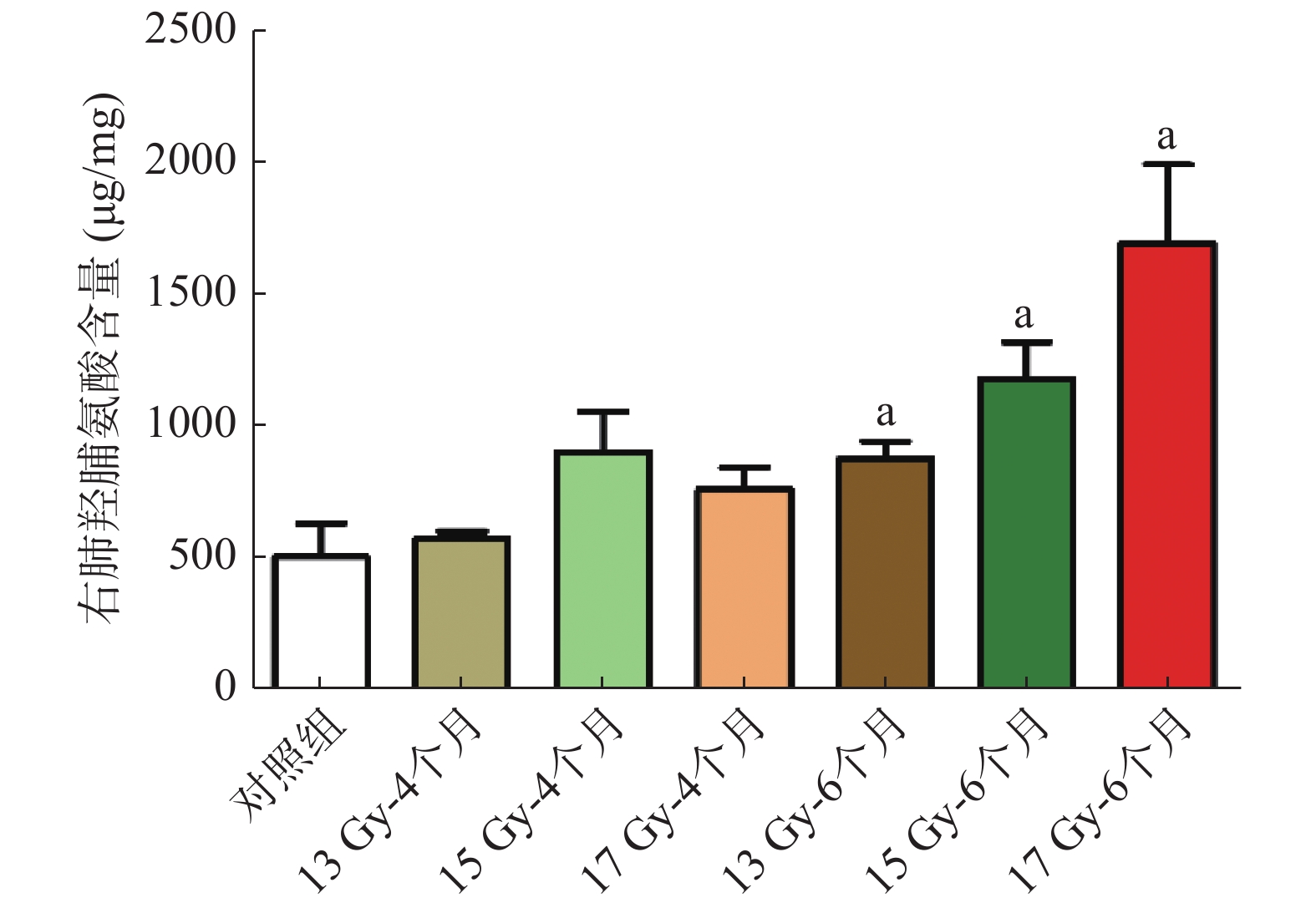
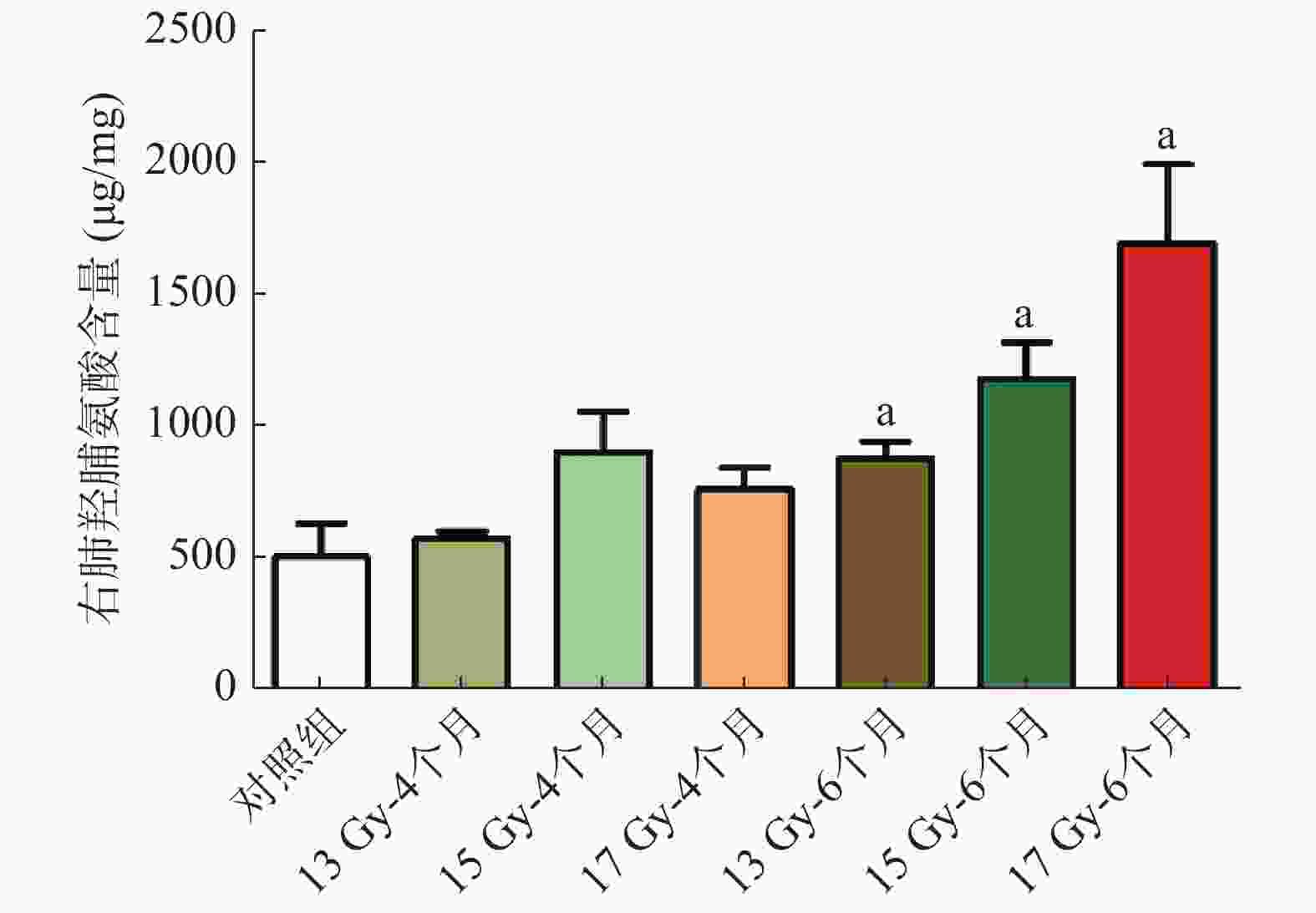
结果显示(图4),与对照组相比,照射后6个月的右肺HYP含量明显增加,分别从(514.19 ±282.20)μg/mg 增加至(886.13 ±145.01)、(1188.70 ±273.84)、(1700.70 ±590.95)μg/mg,差异均有统计学意义(t=2.621、3.609、4.004,均P<0.05)。
-
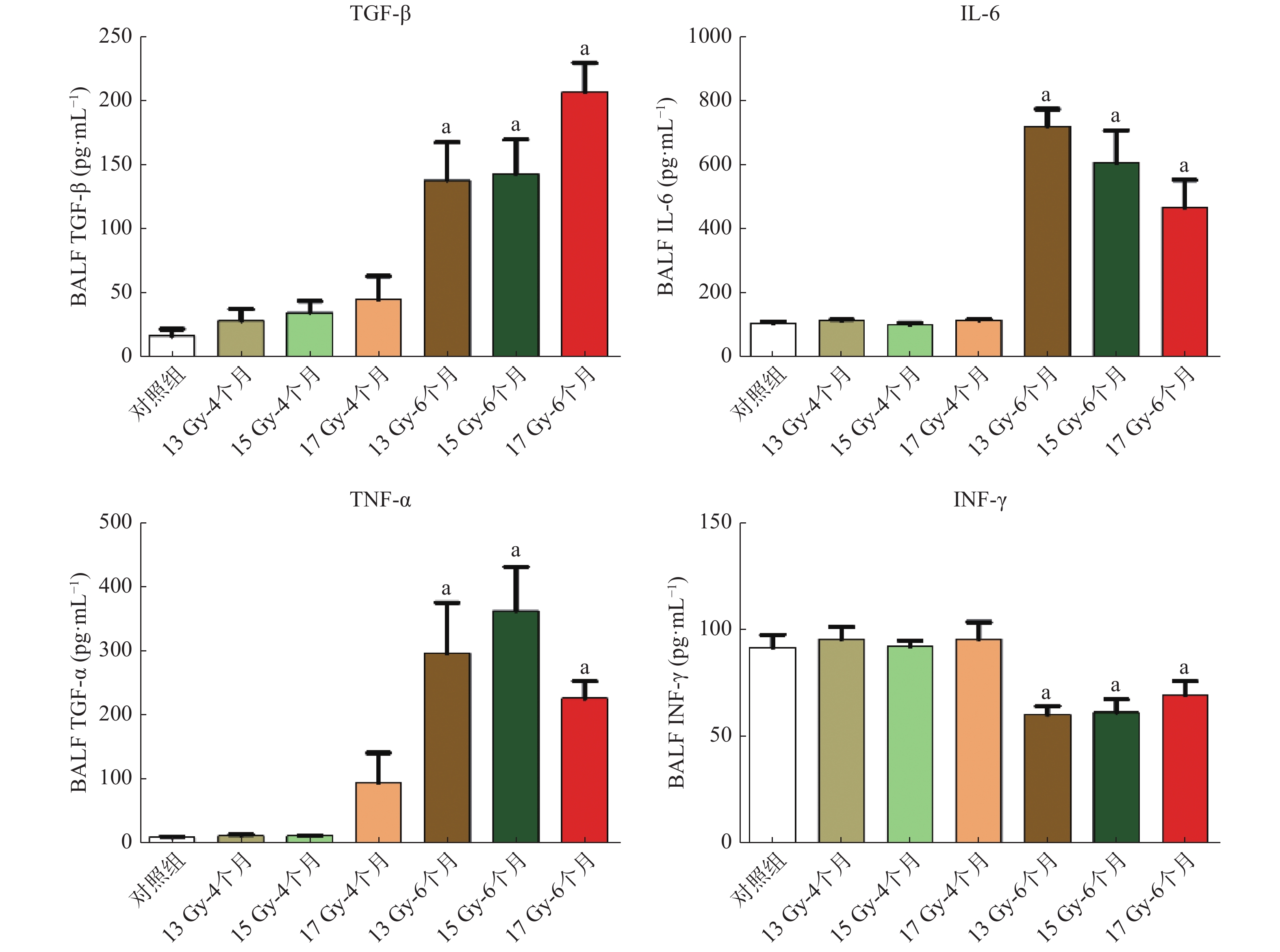
ELISA检测促炎因子TGF-β、IL-6、TNF-α含量变化水平的结果显示,照射后6个月,各照射组与对照组比较,TGF-β分泌量明显增加,差异均有统计学意义(t=4.030、5.128、12.780,均P<0.01);IL-6分泌量明显增加,差异均有统计学意义(t=4.203、4.911、11.160,均P<0.01);TNF-α分泌量明显增加,差异均有统计学意义(t=4.162、5.846、8.129,均P<0.01)。照射后6个月与对照组比较,与抑制胶原合成相关的IFN-γ分泌量降低,差异均有统计学意义(t=4.303、3.537、2.498,均P<0.05)(图5)。
2.1. 一般情况
2.2. 大鼠RIPF模型建立的组织学评估
2.3. Western blot检测结果
2.4. HYP含量测定结果
2.5. RIPF模型鼠BALF中细胞因子的变化
-
RIPF是胸部肿瘤放疗常见的并发症之一,如何减少RIPF的发生,提高放疗疗效,已成为肿瘤放疗研究的热点。本研究旨在建立大鼠RIPF模型,并探寻实验中纤维化蛋白、HYP、细胞因子等做为组织纤维化程度评价指标的可行性,为RIPF的研究提供实验基础。
RIPF的实验动物模型早在上世纪六七十年代就已经成熟建立,随后,不少学者进行了这方面的研究[8]。之前有文献报道,对Wistar大鼠30 Gy全胸单次剂量照射[9],或是对SD大鼠16 Gy全胸单次剂量照射[10],普遍存在放射性肺炎期和RIPF期的动物死亡事件,为保证动物模型检测指标的可信度,只能通过增加样本量来提高实验的可重复性,但样本量的扩大比较耗时耗材。综合考虑模型的成功建立与否和样本量多少,我们对SD大鼠RIPF模型建立的条件进行摸索,采用不同剂量单侧右肺单次剂量的照射方式,复制照射后SD大鼠的RIPF模型,研究照射引起肺损伤的病变规律与特点、病程与分期及检测指标。
本研究构建的动物模型表现出与人类相似的病程变化[11]。受照后1个月出现放射性肺炎,肺泡间隔水肿,表现为单核细胞和成纤维细胞增多,肺泡腔内巨噬细胞增多;受照后4个月出现肺纤维化,肌内膜增生,偶见泡沫细胞,隔膜增厚,气道内充满结缔组织,胶原沉积;受照后6个月肺纤维化程度加重,表现为间质细胞大量增加,肺泡间隔增宽,肺泡完整性下降,胶原沉积严重。以上这些结果表明肺纤维化模型构建成功,可选择单侧右肺17 Gy照射4个月建立RIPF模型。
在RIPF过程中,潜伏的成纤维细胞和(或)周细胞被触发增殖,分化成肌成纤维细胞,并向损伤部位迁移。这些活化的肌成纤维细胞合成并分泌胶原蛋白及其他细胞外基质蛋白[12]。因此,细胞外基质相关蛋白FN1、α-SMA等蛋白表达较正常对照组升高,而降解细胞外基质的MMP2则较正常对照组降低,通过HYP含量测定胶原的结果也显示照射组胶原蛋白比正常对照组升高。鼠类的肺对照射剂量和照射野非常敏感,用相同剂量照射肺部时,肺底较肺尖受到的功能损伤更大。因此,不同部位的取材可能也会影响蛋白的表达。
此外,在受照细胞中也观察到DNA损伤,存在细胞因子和趋化因子的作用,其中较为明确的因子包括IL-1β、IL-6、TNF-α和TGF-β,但在幼龄动物体内,这些标志物可能存在不同[13]。肺部受照后,诱发了慢性氧化应激反应,进而激发了一连串的分子和细胞反应,最终导致肺结构改变和功能丧失。因此,在BALF中,照射组促炎因子的水平较正常对照组升高。IFN-γ主要由淋巴细胞和自然杀伤细胞分泌,下调前胶原基因的表达,抑制胶原的合成,降低成纤维细胞的增殖。因此,照射组IFN-γ较正常对照组降低,进而促进纤维形成。
由于肺纤维化的诱因多种多样,且肺成纤维细胞的活化机制复杂交错,因此在肺纤维化过程中,单一细胞因子会受到其他因素的调节,其变化趋势并不显著。最初,通过比较肺纤维化患者和健康对照组中的细胞因子表达变化发现,在mRNA和(或)蛋白质水平上,不同时间异常升高或降低的细胞因子可作为与肺纤维化过程相关的良好指标[14]。但这些变化不能说明细胞因子是肺纤维化过程的促成因素,因为它们也可能是疾病的结果而不是疾病的原因。细胞因子以促纤维化和抗纤维化方式发挥作用,但靶向或操纵细胞因子尚未使肺纤维化得到有效治疗。因此,细胞因子表达可以是肺纤维化和其严重性的生物标志物,但不是预期的治疗靶标。
利益冲突 本研究由署名作者按以下贡献声明独立开展,不涉及任何利益冲突。
作者贡献声明 李娜负责研究课题的设计、研究过程的实施、数据的获取与分析、论文的撰写;李科君负责实验的实施和数据的分析;王彦、徐畅、纪凯华、何宁宁、王芹、柳杨、刘强负责提出实验的指导方法和论文的审阅;杜利清负责研究课题的提出和论文的最终审阅。









 DownLoad:
DownLoad: