-
肺隐球菌病(pulmonary cryptococcosis,PC)是一种侵袭性肺部真菌病,主要致病菌为新生隐球菌或格特隐球菌,呼吸道吸入是常见的致病途经[1-2]。近年来,PC的发病率呈逐年增加的趋势,据多中心回顾性调查显示,隐球菌已位居侵袭性肺真菌感染常见病原体的第3位[3],而且PC临床症状不典型,影像改变形态各异,可表现为结节肿块型、浸润实变型、弥漫性粟粒型和弥漫混合病变型,其中结节肿块型最多见且具有类似肿瘤的征象,常与肺癌、肺转移癌混淆[4]。本研究回顾性分析22例结节型PC患者的临床及18F-FDG PET/CT显像资料,现将结果报道如下。
-
收集2011年1月至2017年1月于我院手术病理确诊的PC患者22例,其中男性17例、女性5例,年龄(54.77±7.93)岁。就诊原因包括咳嗽、咳痰(伴或不伴胸闷、气促、胸痛)12例,癌胚抗原增高1例,体检发现肺部结节9例。免疫功能受损宿主(immunocompromised host,ICH)9例,免疫受损疾病包括糖尿病3例、乙型肝炎3例(其中1例伴肝癌、肝硬化)、类风湿性关节炎2例、肾病综合征1例,均无人类免疫缺陷病毒感染。非免疫功能受损宿主(non-immunocompromised host,NICH)13例。所有患者均在术前1周内行18F-FDG PET/CT显像,术后病理证实均为PC。所有患者或家属均签署了知情同意书。
-
采用荷兰Philips公司生产的Gemini TF 64 PET/CT仪,18F-FDG由日本住友公司HM-10回旋加速器生产,放射化学纯度>95%。患者检查前禁食6 h以上,控制血糖范围在3.9~7.5 mmol/L,静脉注射185~370 MBq的18F-FDG后,安静休息约60 min,排尿后仰卧位上机扫描。CT扫描采集条件:电压120 kV、电流200 mA,矩阵512×512,层厚5 mm。以1.0 min/床位的速度采集从颅底至股骨上段的PET图像,采用三维模式采集,应用CT数据进行衰减校正。在EBW2.0后处理工作站上,将PET图像和CT图像进行融合,分别得到横断面、矢状面及冠状面的PET、CT和PET/CT融合图像。
-
由两位有5年以上PET/CT诊断经验的核医学科医师共同对PET图像、CT图像及PET/CT融合图像分别判读,包括以下6个方面。(1)病灶数目;(2)病灶大小:手动测量病灶的直径;(3)病灶分布:分为左肺上叶、下叶,右肺上叶、中叶、下叶,肺野内带、中带、外带及胸膜下;(4)SUVmax:选择病灶横断面放射性摄取程度最高层面,以病灶大小的90%为ROI,计算机自动计算SUVmax,并结合目测法,以正常肺野为本底,SUVmax≥2倍肺本底视为18F-FDG代谢增高;(5)结节征象及伴随征象:分叶征、毛刺征、支气管充气征、晕征、胸膜凹陷征、宽基底贴胸膜征、肺门或纵隔淋巴结肿大、胸腔积液等;(6)出现意见分歧以主任医师意见为准。
-
采用SPSS 22.0软件进行统计学分析。符合正态分布的计量资料以
${\bar{x}} \pm s$ -
22例PC患者共发现235个结节,直径(0.7±0.5) cm,范围0.2~2.8 cm。其中,18F-FDG代谢增高结节130个,SUVmax=3.5±2.9(0.9~20.1),直径(0.9±0.5) cm,范围0.3~2.8 cm,二者存在正相关(r=0.702,P=0.000);无18F-FDG代谢结节105个,直径(0.3±0.1) cm,范围0.2~0.6 cm;代谢增高结节的直径大于无代谢者,差异有统计学意义(t=13.621,P=0.000)。
-
以右肺(57.4%,135/235)、下叶(64.3%,151/235)多见结节,多数结节(80.0%,188/235)位于肺野外带或胸膜下,仅少数(11.5%,27/235)位于中带和内带(8.5%,20/235),其余数据见表1。
病灶位置 上叶 中叶 下叶 合计 左肺 35(14.9) 0(0.0) 65(27.7) 100(42.6) 右肺 43(18.3) 6(2.6) 86(36.6) 135(57.4) 合计 78(33.2) 6(2.6) 151(64.3) 235(100.0) Table 1. The distribution of 235 nodules in 22 patients with pulmonary ryptococcosis
-
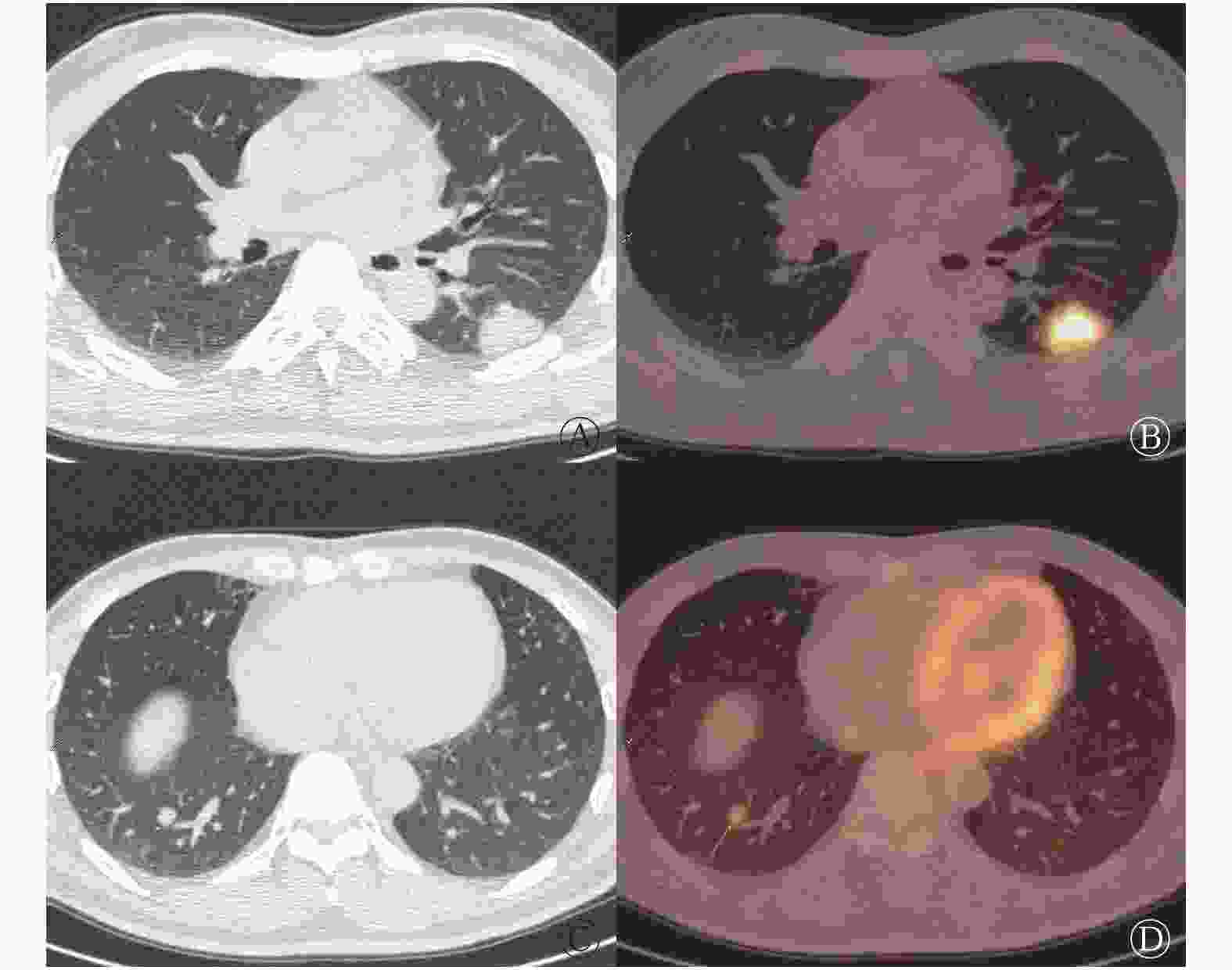
单个肺叶受累者(单发结节8例、单叶散在结节3例)与多个肺叶受累者(多叶散在结节9例、多叶聚集分布结节2例)各11例;PET/CT正确诊断与误诊的患者各11例,其中误诊组中单个肺叶受累者8例。235个结节中出现分叶征、毛刺征、支气管充气征、晕征、空泡征、胸膜凹陷征和宽基底贴胸膜征的个数分别为14、8、28、51、2、5和21,具体数据见表2。典型病例的PET/CT显像见图1,此病例误诊为左侧肺癌右肺转移。
序号 性别 年龄 免疫功能受损 影像诊断 结节分布及数目[a+b(个)] 结节直径(cm) 结节SUVmax 结节征象及个数(个) 右肺上叶 右肺中叶 右肺下叶 左肺上叶 左肺下叶 分叶征 毛刺征 支气管充气征 晕征 空泡征 胸膜凹陷征 宽基底贴胸膜 1 男 56 否 LC 1+0 − − − − 0.6 1.1 − 1 − − − − − 2 男 54 是 LC − 1+0 − − − 2.8 9.6 1 1 1 − − − 1 3 女 53 否 LC 1+0 − − − − 1.4 7.9 1 1 − − − − 1 4 女 49 否 LC − − 1+0 − − 1.5 6.2 1 1 1 − − − − 5 男 43 否 MC − − 1+0 − − 1.6 3.1 − − 1 1 − 1 − 6 男 61 否 MC − − − 1+0 − 1.0 4.3 1 − − 1 − 1 − 7 男 53 否 LC 1+0 − − − − 1.3 5.7 1 1 1 − − − − 8 女 55 是 LC 1+0 − − − − 1.0 7.9 − 1 − − 1 1 − 9 男 54 是 LM − − − − 2+0 0.3/0.5 1.4/3.5 − − − 1 − − − 10 女 69 是 MC − − − − 4+3 0.2~1.6 2.7~13.6 1 − − − − − 1 11 男 57 是 LWM − − − 2+1 − 0.2~1.0 2.5~6.8 1 − − − − − − 12 男 63 是 LWM 4+3 − 0+1 − − 0.3~1.1 2.1~9.3 1 1 − − − 1 − 13 男 41 否 MC − − 12+22 − 20+7 0.3~2.0 1.0~3.8 5 − 17 18 − − − 14 男 35 是 LWM − − 1+0 − 1+0 0.7/2.3 2.3/20.1 − − 1 − − − 2 15 男 60 否 MC 2+1 − 9+1 5+1 − 0.2~1.4 1.8~6.5 − − − − − − 6 16 男 67 是 LWM 2+10 − 2+5 1+3 − 0.2~1.2 1.1~1.7 1 1 1 − − 1 1 17 男 50 否 MC 0+2 4+0 10+2 1+0 6+1 0.3~1.3 0.9~6.8 − − 1 7 − − 1 18 男 57 否 MC 0+6 0+1 4+4 3+10 1+2 0.2~1.2 1.0~6.0 − − 1 5 − − − 19 男 58 否 MC 0+2 − 4+2 − 3+4 0.2~1.5 1.3~6.4 − − 1 3 − − − 20 女 58 是 MC 1+1 − 2+1 − 3+2 0.2~2.3 2.2~10.5 − − 2 5 − − 5 21 男 53 否 MC 2+2 − − 1+3 4+1 0.2~1.5 1.1~7.6 − − − 6 1 − 1 22 男 59 否 MC 1+0 − 1+1 3+0 1+0 0.4~1.7 1.5~12.3 − − − 4 − − 2 注:表中,PC:肺隐球菌病;18F-FDG:氟脱氧葡萄糖F18;PET/CT:正电子发射断层显像计算机体层摄影术;LC:肺癌;MC:真菌感染;LM:肺转移癌;LWM:肺癌伴肺内转移;a:18F-FDG代谢增高结节个数;b:无18F-FDG代谢结节个数;SUVmax:最大标准化摄取值;/:结节数为2枚;~:结节数≥3枚;−:无此项数据 Table 2. The 18F-FDG PET/CT imaging characteristics of 22 patienst with pulmonary cryptococcosis
ICH患者共发现57个结节,18F-FDG代谢增高者25个,SUVmax=5.7±4.7(1.1~20.1);NICH患者共发现178个结节,18F-FDG代谢增高者105个,SUVmax=3.0±2.0(0.9~12.3),二者SUVmax的比较差异有统计学意义(t=2.731,P=0.011)。前者出现宽基底贴胸膜征象的比例高于后者,后者晕征的比例高于前者,差异均有统计学意义。具体数据见表3。
征象 NICH
n/178(%)ICH
n/57(%)χ2值 P值 单叶受累
n/20(%)多叶受累
n/215(%)χ2值 P值 正确诊断
n/191(%)误诊
n/44(%)χ2值 P值 LS 9(5.0) 5(8.8) 1.364 0.243 7(35.0) 7(3.3) 32.911 0.000 7(3.7) 7(15.9) 9.570 0.006 SS 4(2.2) 4(7.0) 3.417 0.065 6(30.0) 2(0.9) 47.022 0.000 0(0.0) 8(18.2) 35.951 0.000 PS 23(12.9) 5(8.8) 0.471 0.492 4(20.0) 24(11.2) 1.362 0.243 23(12.0) 5(11.4) 0.016 0.900 PIS 3(1.7) 2(3.5) 0.836 0.360 3(15.0) 2(0.9) 17.395 0.005 2(1.0) 3(6.8) 5.720 0.047 HS 45(25.3) 6(10.5) 4.628 0.031 3(15.0) 48(22.3) 0.578 0.447 50(26.2) 1(2.3) 12.027 0.000 VS 1(0.6) 1(1.8) 0.832 0.362 1(5.0) 1(0.5) 4.459 0.163 1(0.5) 1(2.3) 1.297 0.340 BBPS 11(6.2) 10(17.5) 7.911 0.005 3(15.0) 18(8.4) 0.988 0.320 16(8.4) 5(11.4) 3.920 0.531 注:表中,18F-FDG:氟脱氧葡萄糖F18;PET/CT:正电子发射断层显像计算机体层摄影术;LS:分叶征;SS:毛刺征;PS:支气管充气征;PIS:胸膜凹陷征;HS:晕征;VS:空泡征;BBPS:宽基底贴胸膜征;NICH:非免疫功能受损宿主;ICH:免疫功能受损宿主 Table 3. The comparison of nodular signs on 18F-FDG PET/CT imaging between different groups
单个肺叶受累者11例共发现20个结节,其中16个18F-FDG代谢增高,SUVmax=5.6±3.4(1.1~13.6);多个肺叶(多叶散在结节)受累者11例共发现215个结节,其中114个18F-FDG代谢增高,SUVmax=3.2±2.7(0.9~20.1),SUVmax差异有统计学意义(t=2.652,P=0.016)。前者出现分叶征、毛刺征、胸膜凹陷征的比例高于后者,差异有统计学意义。具体数据见表3。
18F-FDG PET/CT显像误诊的11例患者共发现44个结节,其中21个18F-FDG代谢增高,SUVmax=5.0±4.6;正确诊断真菌感染的11例患者共发现191个结节,其中109个18F-FDG代谢增高,SUVmax=3.2±2.4,二者SUVmax差异无统计学意义(t=16.825,P=0.106)。前者出现分叶征、毛刺征、胸膜凹陷征的比例高于后者,差异有统计学意义;后者出现晕征的比例高于前者,差异有统计学意义。具体数据见表3。
-
PC是由隐球菌感染引起的一种急性、亚急性或慢性真菌病,呼吸系统是感染的主要门户,好发于中青年男性,本研究中男性患者占多数(17 /22,77.3%),与Severo等[5]报道相符。本研究中54.5%(12/22)的患者主要临床症状为咳嗽、咳痰,而体检发现肺部结节占40.9%(9/22),这说明PC发病隐匿,临床症状轻或无临床症状,这与王丽芳等[6]报道相符。Chang等[1]指出PC好发于免疫功能受损的患者,尤其是HIV感染、使用免疫抑制剂或皮质类固醇治疗的患者,以及有肝炎、糖尿病、结核等基础疾病的患者。但是,近年来免疫功能正常的患者出现隐球菌感染的报道[7-8]并不少见。本研究22例患者均无HIV感染,ICH患者占40.9%(9/22),而NICH的患者占59.1%(13/22),这说明PC在非免疫功能受损人群中也常见,这也与刘又宁等[3]报道一致。
PC的影像学表现各异,临床误诊率高,需与机化性肺炎、肺癌、肺结核等相鉴别[7-9]。目前CT对于PC诊断的报道较多,研究结果显示PC以结节或肿块型多见,分布以肺野外带为主,晕征与支气管充气征对诊断有一定帮助[4, 10-12]。本研究22例PC患者均为结节型,发现的235个结节以右肺、下叶、肺野外带或胸膜下多见,符合PC的分布特征。本研究还显示结节中出现分叶征、毛刺征、支气管充气征、晕征、空泡征、胸膜凹陷征和宽基底贴胸膜征的个数分别为14、8、28、51、2、5和21个,出现晕征的结节个数最多,占21.7%(51/235)。在CT图像上,结节、肿块、实变及空洞等病灶周围环绕密度较淡、均匀的磨玻璃样淡片影,即晕征,病理证实晕征为水肿和(或)出血,欧洲癌症治疗组织及真菌病研究组制定的诊断标准中认为,晕征是肺部真菌感染的特征性表现[13]。杨海等[14]报道,免疫正常患者PC好发于下叶胸膜下,以单发结节和(或)肿块型为主。 本研究正确诊断组中,晕征的比例要高于误诊组,差异有统计学意义,这说明晕征在PC的诊断中是比较可靠的征象;同时在NICH患者中晕征出现的比例略高于ICH,差异有统计学意义,这与兰长青等[12]报道一致。本研究结果显示,单个肺叶受累组以及误诊组中结节出现分叶征、毛刺征及胸膜凹陷征比例高于多个肺叶受累组及正确诊断组,差异有统计学意义,由于误诊组中单个肺叶受累者占72.7%(8/11),所以两组比较结果类似,这也说明单个肺叶受累PC患者结节易出现类肿瘤征象,给诊断带来困扰。冯瑞枝等[15]报道,孤立结节或肿块型PC主要的CT征象为病灶呈宽基底分布并向外紧贴胸膜。本研究中21个结节(21/235,8.9%)出现此征象,仅在ICH组出现的比例高于NICH组,差异有统计学意义,而在其他组别中差异无统计学意义,有待进一步研究。支气管充气征指在病变的肺组织内见透亮的细支气管影,在普通肺炎及肺癌均常见,本研究中该征象在各个组别中的差异均无统计学意义,且本组病例均为结节型,没有出现大片实变或肿块征象。同样,本研究中空泡征在各个组别中的差异均无统计学意义,文献报道PC空洞型少见,多见于免疫缺陷的患者[16],本组病例中仅出现2例,需更大样本量进行研究。本研究并无纵隔或肺门淋巴结肿大以及胸腔积液的病例,说明这两种伴随征象在结节型PC中少见,这与Song等[17]报道类似。
18F-FDG PET/CT作为功能显像与解剖显像融合为一体的影像学检查工具,其在肺结节良恶性鉴别中的应用广泛[18-19],因此18F-FDG PET/CT会在细菌感染(如结核分枝杆菌、真菌等)、放射性炎症、结节病或者手术后局部出现18F-FDG高摄取,出现假阳性[20]。PC作为肺内活动性炎性反应的良性病变也会出现18F-FDG摄取增高,文献多为个案报道[21-24]。本研究中22例PC患者共发现235个结节,18F-FDG代谢增高130个,SUVmax为0.9~20.1,直径(0.9±0.5) cm,明显大于18F-FDG代谢不增高者(0.3±0.1) cm,且SUVmax与直径存在正相关,这与李建南等[25]报道类似。ICH组的SUVmax高于NICH组,差异有统计学意义,这可能与免疫功能受损PC患者炎性细胞活动更活跃有关。PC以单发或多发结节为主,葡萄糖代谢SUV差异大,误诊率较高[26]。单个肺叶受累组中SUVmax高于多个肺叶受累组,这对单个肺叶受累病灶良恶性的判断造成困扰,而且本研究显示误诊组与正确诊断组之间SUVmax的差异无统计学意义,这也说明了18F-FDG代谢增高不能有效提高单个肺叶受累PC患者术前诊断的准确率。
综上所述,PC多见于中青年男性,好发于右肺、下叶、肺野外带或胸膜下。结节SUVmax与直径呈正相关。免疫功能正常的PC患者,结节易出现晕征,在多个肺叶受累者中,晕征是可靠的征象,但对于单个肺叶受累者,PC的类肿瘤征象及增高的SUVmax使得18F-FDG PET/CT显像易误诊,需引起临床重视。
利益冲突 本研究由署名作者按以下贡献声明独立开展,不涉及任何利益冲突。
作者贡献声明 李生栩负责命题的提出与设计、论文的撰写;唐明灯负责论文的修订与审校、图像的分析;林端瑜负责图像的分析、英文的校对;刘道佳、张杰平、吕清湖负责图像的采集、数据的统计分析。
Retrospective analysis of 18F-FDG PET/CT imaging in 22 cases of nodular type pulmonary cryptococcosis
- Received Date: 2019-03-19
- Available Online: 2020-01-01
Abstract:








 DownLoad:
DownLoad: