-
心血管疾病严重威胁着全球人类的健康,据报道,2017年全球范围内约1700万人病死于心血管疾病,而其中有3/4在发展中国家[1]。我国心血管疾病患者目前有2.9亿,因此对于心血管疾病的早诊断和防治是刻不容缓的[2]。随着影像技术的迅猛发展,冠状动脉CT血管造影(coronary computed tomography angiography,CCTA)已经成为冠状动脉疾病危险因素的临床评估标准,并且已应用于冠状动脉支架植入患者的随访中,以评估植入支架的通畅性及判断是否有相关并发症[3]。但是,CCTA对心血管疾病的诊断严重依赖于人工图像后处理质量以及诊断医师的经验,尤其我国的医疗资源严重紧缺,对所有心血管疾病患者均行CCTA临床评估是难以实现的。
人工智能(artificial intelligence,AI)通俗来讲就是模仿人类思维,是具有“认知”功能的机器,其通过“学习”来“解决问题”。AI作为21世纪三大尖端技术之一,在众多领域均获得了广泛的应用,并取得了丰硕的成果。AI在医疗领域可用于计算患者给药剂量、肿瘤药物的选择、高危患者的监测,甚至手术的实施,且CT图像报告解读更是AI的优势所在[4]。将AI应用于CCTA图像后处理以及诊断报告的书写,势必能够缓解我国紧张的医疗资源,但是冠状动脉AI对于CCTA图像处理、诊断报告的准确性以及是否能够代替临床医师,目前尚不明确。本研究从冠状动脉AI与人工后处理、诊断报告等方面分别进行对比,以此评估冠状动脉AI在CCTA的应用前景。
HTML
-
选取重庆医科大学附属第三医院自2019年4月至7月就诊的疑似冠心病患者64例,其中男性40例、女性24例,年龄(62.16±14.13)岁。纳入标 准:心率正常或者轻度心率不齐的疑似冠心病患 者。排除标准:严重心律不齐且伴心率>200次/min;对于碘剂过敏;不能配合完成检查。
-
所有患者均使用美国GE公司的revolution CT行图像采集,选用非离子型造影剂(320 mg I/mL,江苏恒瑞医药股份有限公司)50 mL,生理盐水20 mL,流速5 mL/s;对比剂示踪法选择主动脉根部ROI监测CT值(触发扫描CT值设为150 HU),延迟4 s,采用回顾性心电门控法进行扫描。扫描参数:管电压120 kV,管电流20 mAs,层厚0.625 mm,总扫描时间0.3 s。随后使用冠状动脉AI(数坤科技的冠心病智能辅助诊断系统,其主要功能是全自动完成冠状动脉影像图像后处理、AI 辅助冠状动脉诊断结果和胶片智能打印,并输出结构化报告)进行图像后处理及影像诊断,分别从容积再现、曲面重建及AI诊断报告进行分析。CT增强检测患者均签署了知情同意书。
-
扫描完成后,按照李克特量表(Likert scale)评分标准,将原始图像质量由低到高进行评分(1~5分)。1分:差,图像质量过度受损,血管壁不能辨认;2分:较差,图像质量低,血管边界不清晰或伪影过大,对比度分辨率低;3分:中,存在一定图像伪影,但对比度分辨率较高,血管边界较清晰;4分:良,血管边界和血管壁清晰,图像伪影很小,对比度分辨率高;5分:优,管壁显示清晰,分辨率好,没有伪影。对冠状动脉图像分别进行人工和AI图像后处理,参照美国心脏病协会(SCCT)冠状动脉分段法将冠状动脉分为18段[5],判断冠状动脉三大支:左前降支、回旋支和右冠状动脉狭窄部位及斑块性质。分别由两位医师(住院医师和副主任医师)进行图像人工后处理及诊断报告,最终结果由两位副主任医师进行审核。
1.1. 一般资料
1.2. 检查方法及AI的应用
1.3. 图像分析
-
在64例患者中,5例患者AI冠状动脉重建失败,余59例患者重建图像合格,其中男性36例、女性23例,年龄(62.75±14.32)岁。
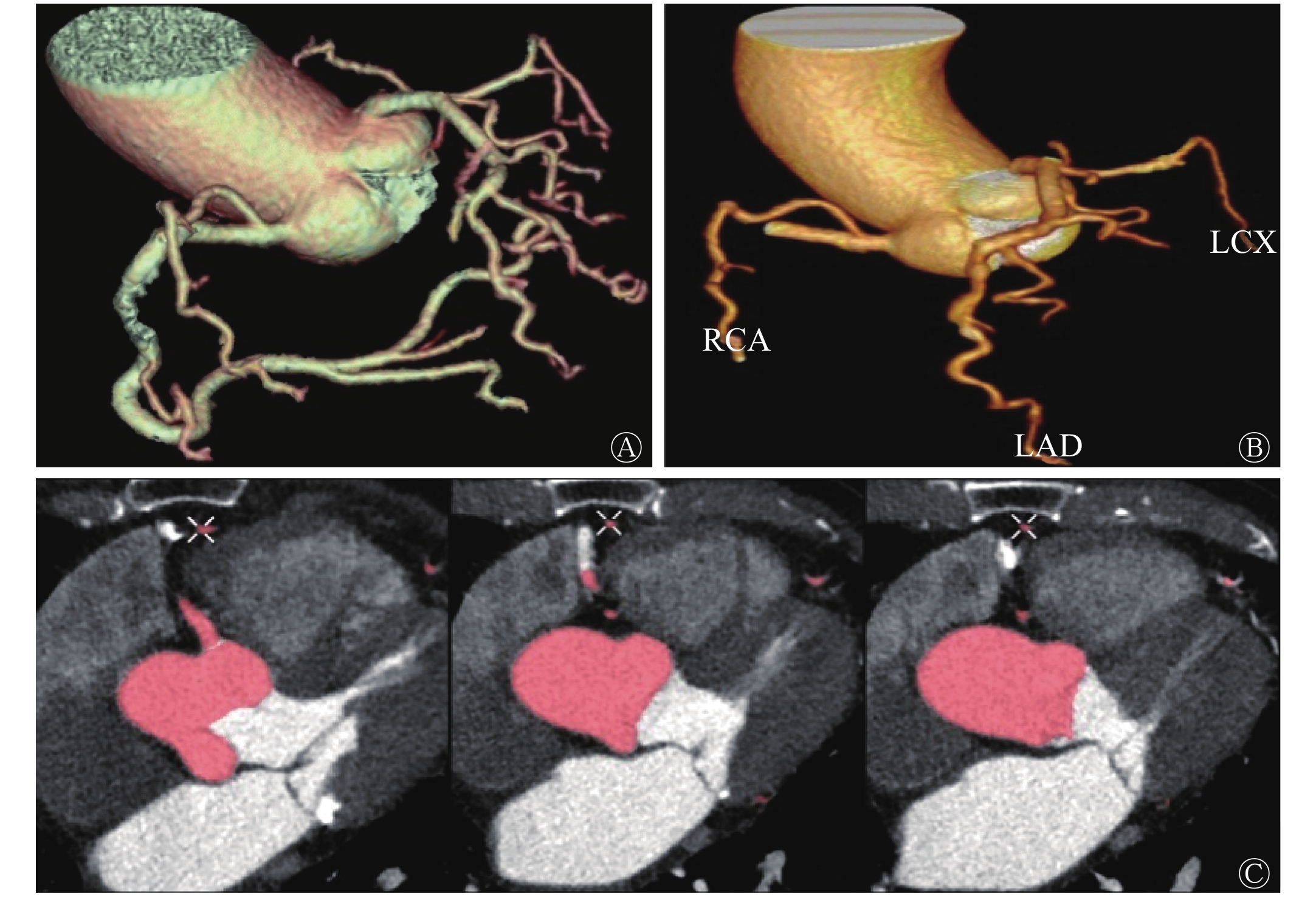
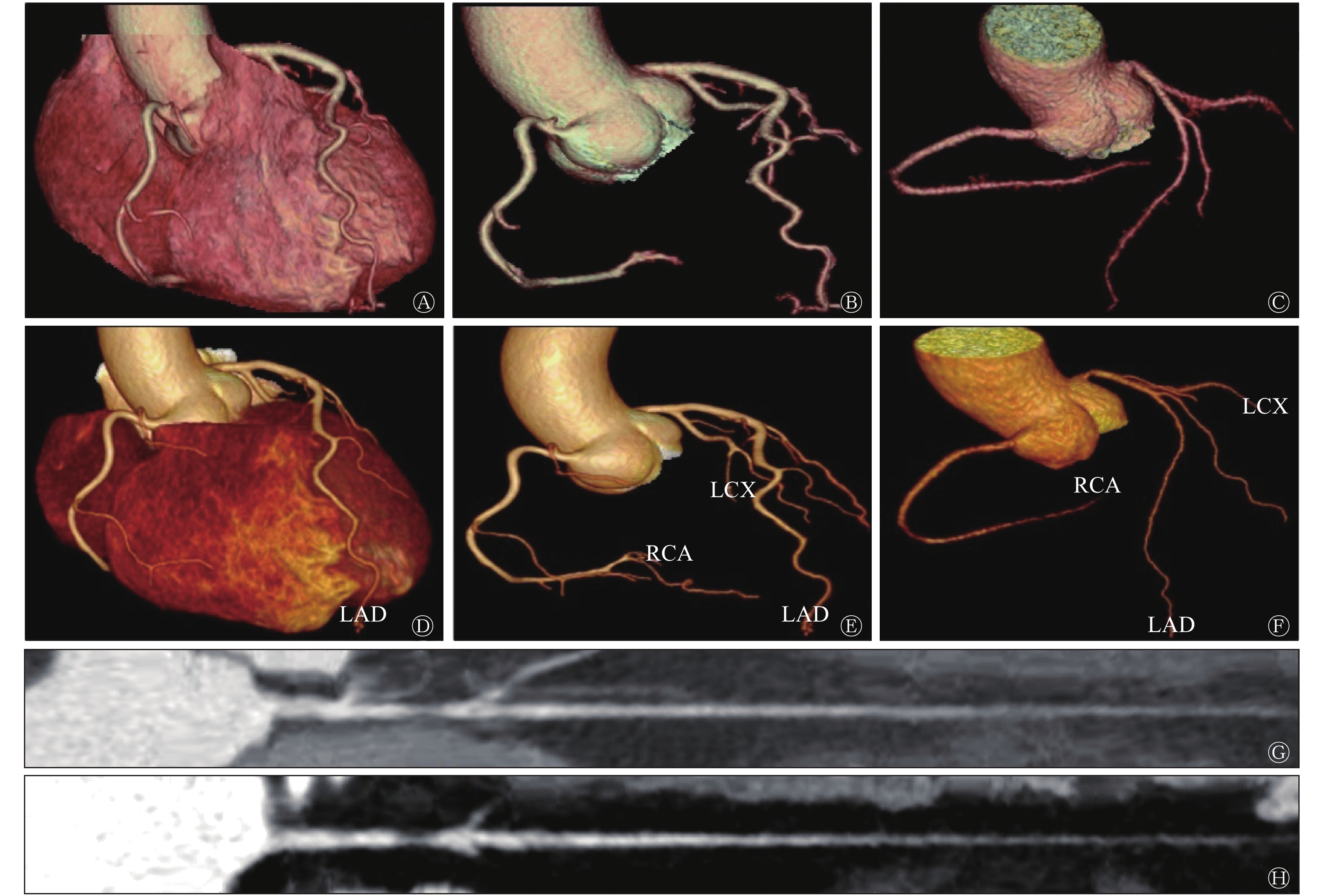
冠状动脉AI图像后处理耗时约3 min,而人工图像后处理时间约为20~30 min,AI后处理耗时约为人工耗时的10%,并且冠状动脉AI后处理的合格率为92.2%(59/64)。如图1所示,冠状动脉AI图像后处理能够对各支血管进行自动命名。AI后处理(图1中D~F)与人工后处理(图1中A~C)相比,冠状动脉图像分支更多、更长、管壁更光滑、细节小分支显示更全面;在冠状动脉拉直图像中,发现冠状动脉AI处理的图像(图1中H)较人工处理图像(图1中G)的血管更加清晰,并且能够自动识别冠状动脉狭窄。此外,本研究还发现冠状动脉AI也能够自动识别植入的冠状动脉支架,这都暗示着冠状动脉AI在图像后处理中的应用价值。
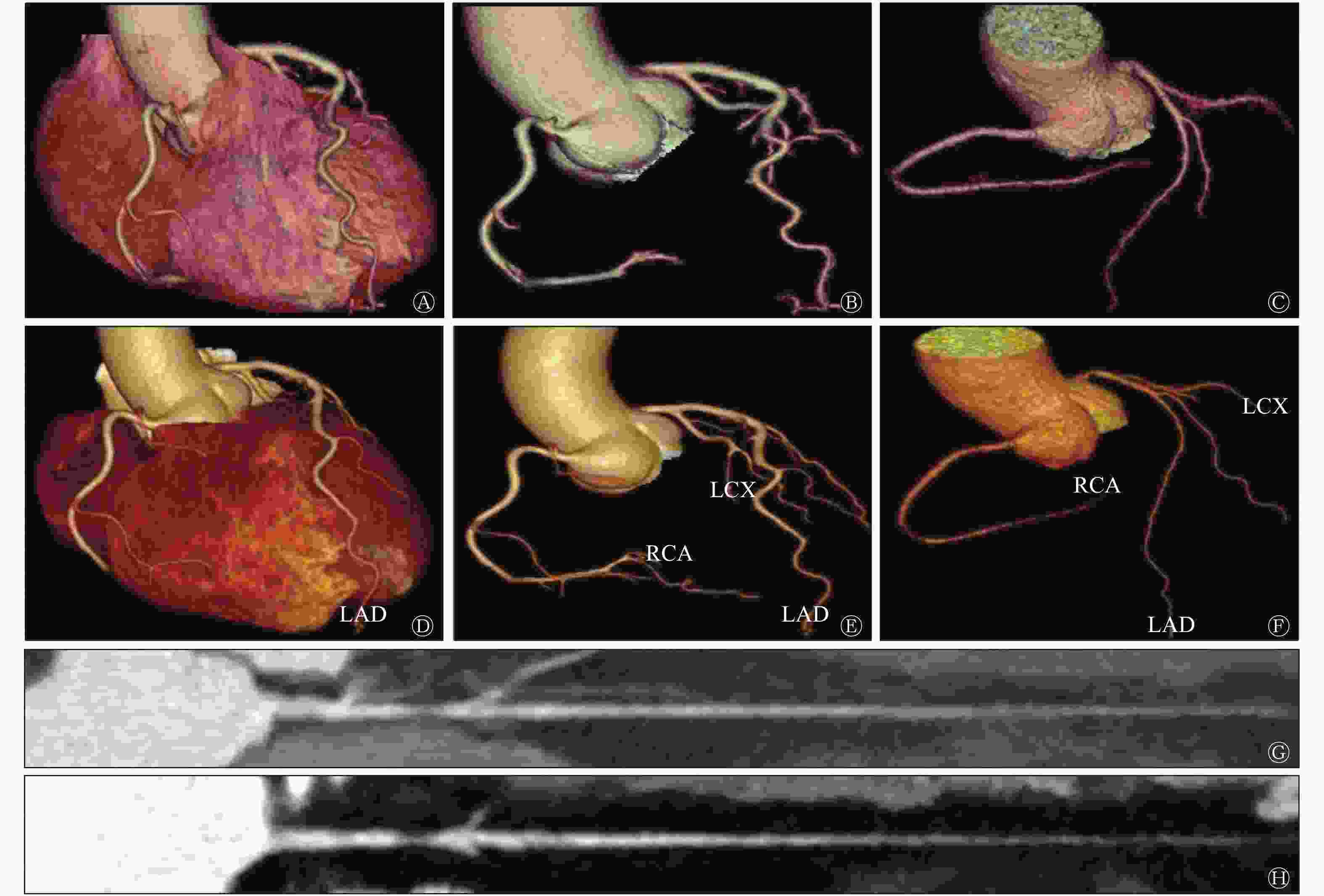
Figure 1. The comparison between artificial intelligence post-processing image and artificial post-processing image of coronary artery after CT enhanced scan
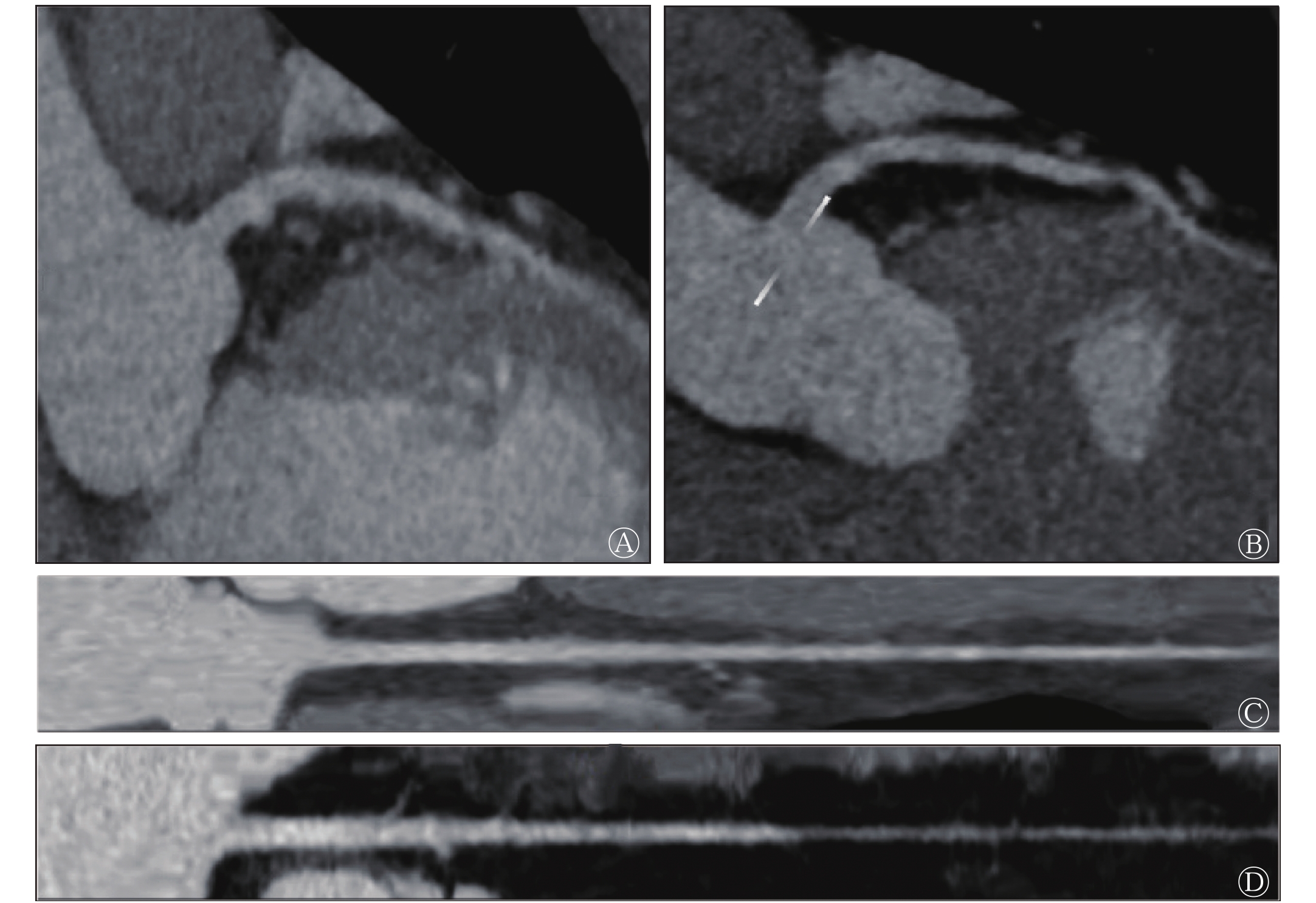
虽然冠状动脉AI在图像后处理上表现优异,但本研究同时发现,冠状动脉AI图像后处理有不足之处。如表1所示,冠状动脉AI图像后处理的合格率为92.2%(59/64),原始图像质量为1分及2分的病例共有5例,AI后处理全部为不合格;而图像质量达到3分时,AI图像后处理合格率达到100%。AI后处理图像(图2中B)与人工后处理图像(图2中A)相比,AI分割遗漏导致右冠状动脉血管截断、命名错误,无法全面显示血管,其后处理失败的原因之一为钙化斑块周围伪影导致管腔被掩盖(图2中C),而人工后处理可以通过工作站手动添加使截断血管“再生”。这也就体现出冠状动脉AI图像后处理严重依赖原始图像质量的弊端。
项目 图像质量等级(分) 5 4 3 2 1 患者(例) 45 10 4 3 2 患者比例(%) 70.31 15.62 6.25 4.69 3.12 人工智能后处理的合格率(%) 100 100 100 0 0 注:表中,CT:计算机体层摄影术 Table 1. The original image quality score of 64 patients and the corresponding qualified rate of coronary artificial intelligence post-processing after CT enhanced scan
-
冠状动脉AI图像的诊断报告在图像重建后即可完成(<1 min),而人工出具一份冠状动脉的诊断报告通常需要耗时15 min左右。与AI后处理一样,AI在报告的诊断上同样高效。如表2所示,冠状动脉AI斑块检出的灵敏度达93.3%,与人工报告的灵敏度(92.0%)相当。本研究中,人工报告对斑块的检出不存在假阳性结果,其特异度达100%,而冠状动脉AI对其检测的特异度为93.8%(表3)。3支分支血管中,冠状动脉AI对左前降支病变的特异度最低(88.0%)。
斑块
位置人工 人工智能 真阳性(个) 假阴性(个) 灵敏度(%) 真阳性(个) 假阴性(个) 灵敏度(%) 左前降支 37 4 90.2 40 1 97.6 回旋支 11 1 91.7 11 1 91.7 右冠状动脉 21 1 95.4 19 3 86.4 总计 69 6 92.0 70 5 93.3 Table 2. Sensitivity of artificial intelligence and the artificial to detection of coronary plaque
斑块位置 真阴性(个) 假阳性(个) 特异度(%) 左前降支 22 3 88.0 回旋支 45 2 95.7 右冠状动脉 38 2 95.0 总计 105 7 93.8 Table 3. Specificity of artificial intelligence for detection of coronary plaque
本研究结果发现,合格的后处理图像并不代表正确的AI诊断报告,如图3中A、B所示,虽然冠状动脉AI将心肌桥成功处理,但是诊断报告并未提及。从图3中C、D发现,冠状动脉AI将分布在左前降支较细管腔内的造影剂误报为钙化斑块,其他的还有管壁毛糙造成的非钙化斑块的误报。
2.1. 冠状动脉AI与人工的图像后处理比较
2.2. 冠状动脉AI与人工的诊断报告比较
-
通过对64例疑似冠心病患者的CCTA的研究发现:(1)冠状动脉AI图像后处理的工作效率是人工的10倍左右,AI后处理的冠状动脉分支更多、更长、管壁更光滑,并且能够识别冠状动脉狭窄及冠状动脉支架,后处理合格率为92.2%。(2)在诊断报告书写方面,冠状动脉AI的工作效率是人工书写报告的15倍左右,冠状动脉AI对斑块检出的灵敏度为93.3%、特异度为93.8%。
AI技术的迅猛发展,使其在医疗领域的运用已被开发,并得以应用,如疾病的诊断、治疗方案的制定、个性化医疗服务以及重症患者的监测和护理等。在国外梅奥诊所、麻省总医院等医疗机构都研发了各自的AI算法并应用于临床[6]。在影像学方面,斯坦福大学研究并创造了一种新的算法,其检测特定部位肺炎的表现优于经验丰富的临床医师[7]。本研究结果发现,冠状动脉AI的影像诊断报告已经达到与人工报告相当的灵敏度,并且能够发现人工报告中遗漏的微小钙化斑块。鉴于AI在放射学领域的出色表现,一些专家认为AI的出现是一种威胁[8],因为与专家相比,AI基于统计学习指标的改进,随着深度地学习罕见病例,其对个案的分析更具优势[9-10]。
与国内外研究结果相比,本研究的冠状动脉AI对斑块检出的灵敏度为93.3%、特异度为93.8%,显著高于黄增发和王翔[11]的研究结果(灵敏度为54.35%、特异度为81.73%),其原因可能是我们的冠状动脉AI深入学习的熟练度更高、技术更加完善。虽然既往研究报道,通过深度学习的冠状动脉AI计算出的钙化积分广泛得到专家的认可[12-13],但是本研究冠状动脉AI对斑块的检出仍存在遗漏(主要是部分不明显小钙化斑块的遗漏)。
本研究发现冠状动脉AI存在诸多不足之处:(1)图像重建方面。在纳入的64例患者中,有5例的冠状动脉AI由于其伪影、分割遗漏导致血管截断和命名错误,我们认为造成这种结果的原因是原始图像质量较差(5例患者Likert评分为1~2分),因此冠状动脉AI的图像重建较人工图像处理更加依赖高质量的原始图像,较人工后处理应变能力差。(2)诊断报告方面。冠状动脉AI较人工报告更容易误报软斑及钙化斑块,这是造成其特异度低的主要原因:①当图像出现伪影时,AI将其误报非钙化斑块;②当造影剂在较细管腔内分布不均时,AI将造影剂误认为钙化斑块。因此,冠状动脉AI正确的后处理图像,并不一定是正确的诊断报告。同时,本研究也有一些不足之处:(1)本研究是一个单中心的研究,病例集中为重庆及其周边地区的患者,而冠状动脉AI的深度学习是建立在多中心的基础上,因此其诊断效能受到影响;(2)受限于冠状动脉AI在我院应用时间较短,病例总数偏少,后期随着冠状动脉AI在多中心的不断深入学习,我们一定能够得到更加完善的数据。
综上所述,虽然AI在CCTA乃至整个影像方面取得了令人震惊的成果,其整体水平表现优异,尤其是在SPECT心肌核素显现中的应用[14-15],但是AI在影像中的应用同样也有其弊端,美国食品药物管理局仍将AI应用于辅助诊断归为2类(中危)设备,而将AI应用于诊断报告归为3类(高危)设备[16]。因此,一份合格的影像报告不能仅仅依靠AI后处理及诊断,从根本上需要依赖于影像医师的把控。我们认为AI技术并非我们影像医师的终结者,而是我们诊断疾病的一个有效的利器。
利益冲突 本研究由署名作者按以下贡献声明独立开展,不涉及任何利益冲突。
作者贡献声明 胡小丽负责数据的收集与分析、论文的撰写;向守洪、胡荣慧负责诊断报告的书写;周代全、陈俊源负责图像的采集及后处理;薛雨负责数据的统计;王政杰负责提供论文思路与修订。









 DownLoad:
DownLoad: