-
弥漫大B细胞淋巴瘤(diffuse large B-cell lymphoma, DLBCL)是非霍奇金淋巴瘤(non-Hodgkin lymphoma,NHL)中最常见的病理类型,约占成人NHL的1/3[1]。18F-FDG PET/CT作为一种新型分子影像技术,在淋巴瘤尤其是DLBCL疗效评价及预后判断中具有重要的价值[2]。但是既往大部分研究中包含了Ⅰ~Ⅳ期的DLBCL患者,而Ⅰ期患者的预后本身就好于Ⅳ期的患者。因此,本研究回顾性分析85例Ⅱ~Ⅲ期DLBCL患者的临床资料和18F-FDG PET/CT显像资料,旨在探讨治疗前18F-FDG PET/CT显像中PET代谢参数的预后价值。
HTML
-
收集2009年6月至2015年12月在南京医科大学第一附属医院诊治且行18F-FDG PET/CT检查的85例Ⅱ~Ⅲ期DLBCL患者,其中男性35例、女性50例,中位年龄55(20~78)岁。入组标准:①年龄≥18岁;②病理确诊为DLBCL,Ann Arbor分期[3]为Ⅱ期或Ⅲ期;③PET/CT检查前未接受任何抗肿瘤治疗;④化疗前PET/CT检查可见淋巴瘤阳性病灶;⑤化疗方案为利妥昔单抗+环磷酰胺+阿霉素+长春新碱+泼尼松(R-CHOP);⑥病历资料完整;⑦无其他恶性肿瘤病史。排除标准:①伴有活动性感染或不可控制的慢性感染;②伴有严重的心、脑血管疾病,如难治性心功能不全等。所有患者于PET/CT检查前均签署了知情同意书。
-
采用德国西门子公司生产的Biograph 16 HR PET/CT扫描仪,18F-FDG由美国GE公司生产的PET trace型回旋加速器生产并通过自动化合成模块自动合成,放射化学纯度>95%。检查前所有患者至少空腹6 h,并控制空腹血糖≤7.0 mmol/L,经静脉注射18F-FDG 3.70~5.55 MBq/kg后安静休息40~60 min,排尿后行PET/CT扫描。先行CT扫描,扫描参数:管电压120 kV,管电流140 mA,层厚3.75 mm,准直1.5 mm。再行PET数据采集:采用三维模式采集6~7个床位,每个床位采集2~3 min。应用CT数据进行衰减校正,迭代法重建获得融合图像。
-
通过PET/CT数据处理工作站,采用麦迪克斯软件对数据进行处理。采用相对阈值法,参照文献[4−5]并结合本研究,将SUVmax大于1.5倍肝脏平均标准化摄取值(mean standardized uptake value, SUVmean)+2倍肝脏SUVmean标准差的病灶设为可测量病灶,将上述病灶包括在容积感兴趣区内(volume of interest, VOI),以SUVmax的41%作为阈值,由软件自动进行容积分割,得出病灶的SUVmax、代谢体积(metabolic tumor volume, MTV)及病灶糖酵解总量(total lesion glycolysis, TLG)。
-
采用门诊进行随访,随访时间为2009年6月1日至2018年1月1日,无患者失访。无进展生存期(progression-free survival, PFS)为疾病诊断至第一次出现疾病复发、进展、疾病所致死亡或随访截止时间。总生存期(overall survival, OS)为疾病诊断至疾病所致死亡或随访截止时间。
-
应用SPSS 17.0软件进行统计学分析。符合偏态分布的计量资料以中位数(四分位间距)表示,采用受试者工作特征(receiver operating characteristic , ROC)曲线获得SUVmax、MTV及TLG的最佳界值点,并计算曲线下面积(area under the curve, AUC);如果AUC较小,则以中位数进行分组。采用Kaplan-Meier法进行生存分析,单因素分析采用Log-rank 检验,多因素分析采用Cox比例风险模型。P<0.05为差异有统计学意义。
1.1. 一般资料
1.2. PET/CT检查方法
1.3. 图像分析
1.4. 随访
1.5. 统计学方法
-
经过中位随访46(24~102)个月,85例患者中,26例患者复发或进展,中位PFS为31.0(2.5~99.7)个月,1年、3年无进展生存率分别为83.5%、40%;15例患者死亡,中位OS为34.8(2.0~99.7)个月,1年、3年总生存率分别为91.8%、48.2%。
-
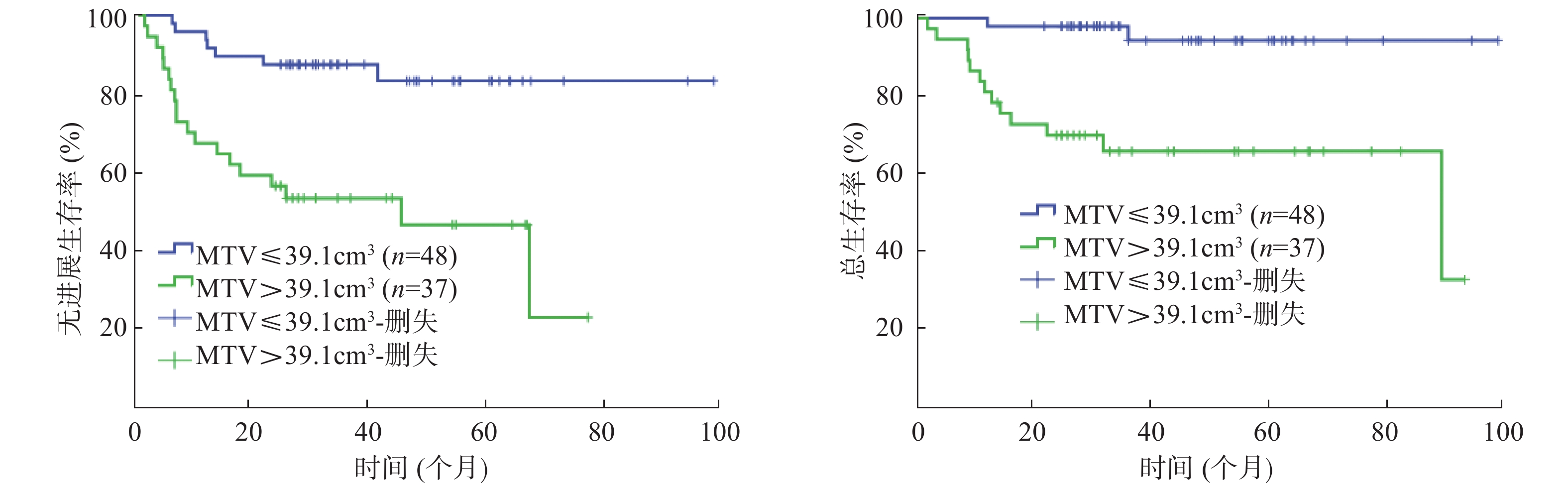
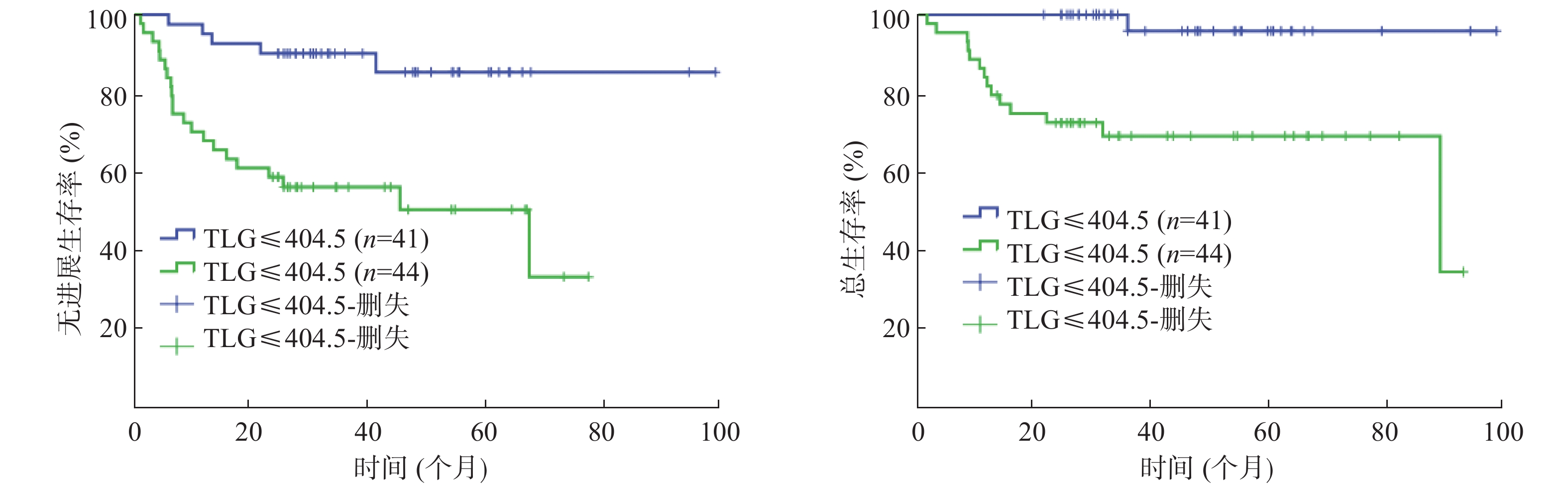
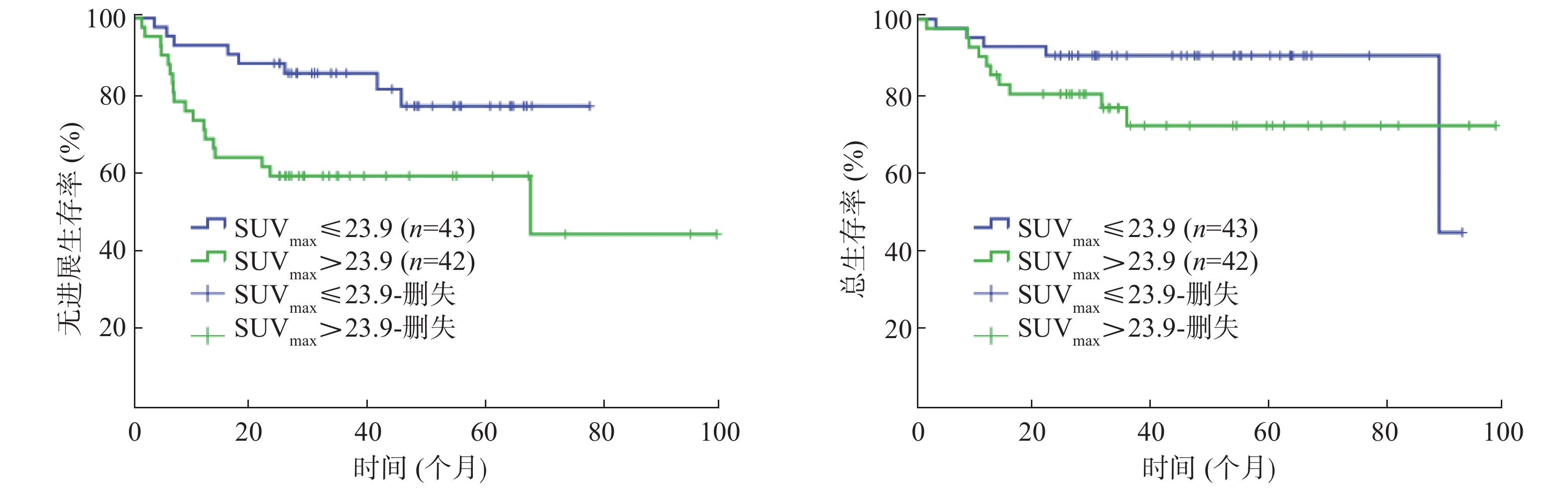
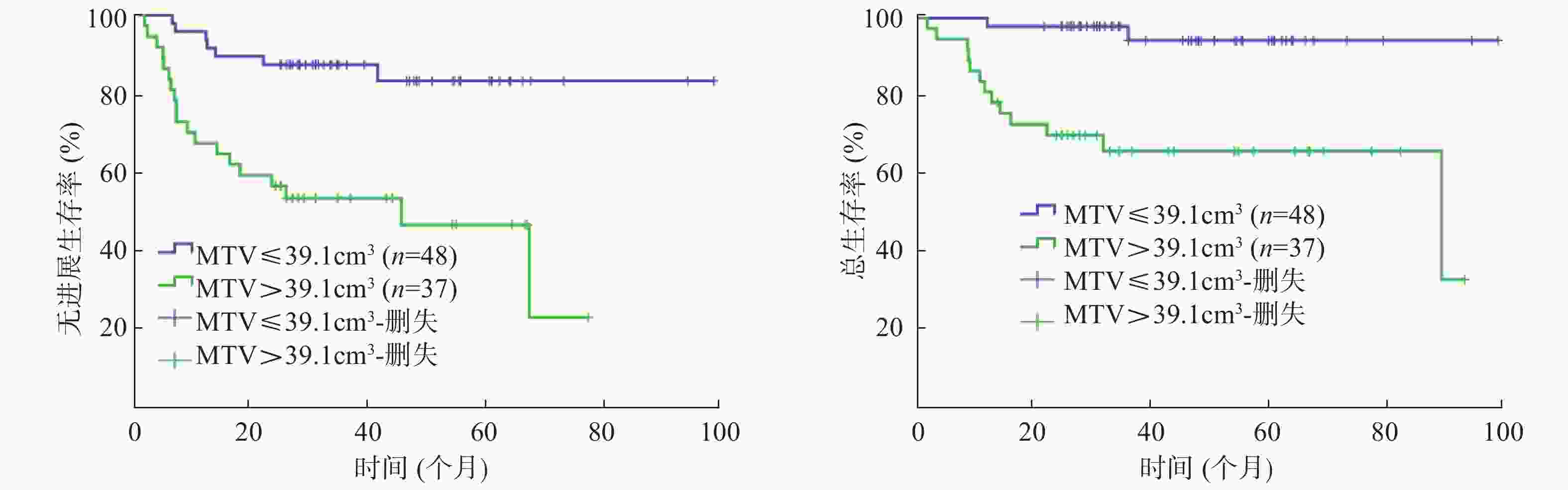
85例患者的SUVmax、MTV、TLG的中位数分别为23.9(16.3)、25.1(95.3)cm3、424.4(1404.6)。以复发或进展作为阳性事件,ROC曲线分析结果显示:SUVmax的AUC=0.610(95%CI=0.483~0.736,P=0.109),MTV 的AUC=0.729(95%CI=0.621~0.838,P=0.001),TLG 的AUC=0.726(95%CI=0.621~0.831,P=0.001)。由于SUVmax的AUC较小,不能通过ROC曲线分析获得界值点,因此,以SUVmax的中位数23.9作为界值点进行分组。MTV的界值点为39.1 cm3(灵敏度为73.1%,特异度为69.5%);TLG的界值点为404.5(灵敏度为80.8%,特异度为61.0%)。
-
单因素分析结果显示:Ann Arbor分期、β-2微球蛋白、乳酸脱氢酶(lactate dehydrogenase, LDH)、大肿块、国际预后指数(international prognosis index, IPI)、SUVmax、MTV、TLG均是Ⅱ~Ⅲ期DLBCL患者PFS的影响因素(χ2=19.118、12.310、4.861、7.731、19.693、6.414、14.538、13.089,均P<0.05);Ann Arbor分期、β-2微球蛋白、大肿块、IPI、MTV、TLG 是患者 OS 的影响因素(χ2=14.550、5.473、5.643、15.943、13.877、12.677,均P<0.05)(表1,图1~3)。
因素 例数 中位无进
展生存
期(个月)χ2值,P值 中位总
生存期
(个月)χ2值,P值 因素 例数 中位无进
展生存
期(个月)χ2值,P值 中位总
生存期
(个月)χ2值,P值 性别 乳酸脱氢酶 男 35 31.0 χ2=0.734, 33.5 χ2=0.539, 正常 54 32.4 χ2=4.861, 34.8 χ2=2.150, 女 50 28.0 P=0.392 39.4 P=0.463 升高 31 26.0 P=0.027 33.2 P=0.147 年龄 大肿块 ≤60岁 53 32.8 χ2=1.656, 35.0 χ2=1.759, 无 64 33.8 χ2=7.731, 37.0 χ2=5.643, >60岁 32 25.5 P=0.198 30.5 P=0.185 有 21 25.0 P=0.013 26.0 P=0.018 B症状 国际预后指数 无 67 31.0 χ2=2.557, 34.8 χ2=0.074, 低危组(0~1分) 46 36.4 χ2=19.693, 43.2 χ2=15.943, 有 18 28.0 P=0.108 33.2 P=0.786 中高危组(2~4分) 39 24.1 P=0.000 28.0 P=0.000 ECOG评分 SUVmax 0~1分 76 31.0 χ2=0.899, 35.0 χ2=0.383, ≤23.9 43 36.4 χ2=6.414, 39.4 χ2=2.158, 2~3分 9 24.1 P=0.343 28.0 P=0.536 >23.9 42 26.0 P=0.011 29.0 P=0.142 Ann Arbor分期 MTV(cm3) Ⅱ期 45 34.8 χ2=19.118, 39.4 χ2=14.550, ≤39.1 48 34.7 χ2=14.538 43.7 χ2=13.877, Ⅲ期 40 25.0 P=0.000 28.0 P=0.000 >39.1 37 25.0 P=0.000 28.0 P=0.000 病理亚型 TLG 生发中心 39 33.8 χ2=1.650, 37.0 χ2=0.099, ≤404.5 41 36.4 χ2=13.089, 45.7 χ2=12.677, 非生发中心 46 30.5 P=0.199 31.0 P=0.735 >404.5 44 25.9 P=0.000 29.0 P=0.000 β-2微球蛋白 正常 68 33.8 χ2=12.310, 36.4 χ2=5.473, 升高 17 22.0 P=0.000 25.0 P=0.019 注:表中,DLBCL:弥漫大B细胞淋巴瘤;ECOG:美国东部肿瘤协作组;SUVmax:最大标准化摄取值;MTV:代谢体积;TLG:病灶糖酵解总量。 Table 1. Univariate analysis for survivals of 85 patients with diffuse large B cell lymphoma of stage Ⅱ~Ⅲ disease

Figure 1. Kaplan-Meier curves for progression-free survival(A) and overall survival(B) of patients 85 patients with diffuse large B cell lymphoma of stage Ⅱ~Ⅲ disease by SUVmax
-
由于MTV与TLG呈高度相关,参照文献[6]并结合本研究,多因素分析时,二者中仅纳入了TLG,结果显示:LDH、TLG是影响Ⅱ~Ⅲ期DLBCL患者PFS的独立危险因素(RR=4.891, 95%CI=1.332~11.955,P=0.017;RR=0.195, 95%CI=0.058~0.660,P=0.009)(表2),IPI、TLG是影响患者OS的独立危险因素(RR=0.508, 95%CI=0.270~0.956,P=0.036;RR=0.433, 95%CI=0.227~0.826,P=0.011)(表3)。
因素 β Wald值 RR 95%CI P值 Ann Arbor分期 −1.230 3.247 0.292 0.077~1.114 0.072 β-2微球蛋白 −0.696 2.816 0.499 0.221~1.124 0.093 LDH 1.587 5.723 4.891 1.332~11.955 0.017 大肿块 −0.818 3.288 0.441 0.182~1.068 0.070 IPI −1.232 2.370 0.292 0.061~1.400 0.124 SUVmax −0.746 2.168 0.474 0.176~1.280 0.141 TLG −1.635 6.898 0.195 0.058~0.660 0.009 注:表中,DLBCL:弥漫大B细胞淋巴瘤;PFS:无进展生存期;LDH:乳酸脱氢酶;IPI:国际预后指数;SUVmax:最大标准化摄取值;TLG:病灶糖酵解总量。 Table 2. Multivariate analysis for progression-free survival of 85 patients with diffuse large B cell lymphoma of stage Ⅱ~Ⅲ disease
因素 β Wald值 RR 95%CI P值 Ann Arbor分期 0.073 0.063 1.076 0.610~1.897 0.801 β-2微球蛋白 −0.115 0.143 0.892 0.492~1.616 0.706 大肿块 0.295 0.868 1.343 0.722~2.499 0.351 IPI −0.678 4.406 0.508 0.270~0.956 0.036 TLG −0.837 6.443 0.433 0.227~0.826 0.011 注:表中,DLBCL:弥漫大B细胞淋巴瘤;OS:总生存期;IPI:国际预后指数;TLG:病灶糖酵解总量。 Table 3. Multivariate analysis for overall survival of 85 patients with diffuse large B cell lymphoma of stage Ⅱ~Ⅲ disease
2.1. 患者生存情况
2.2. PET代谢参数判断预后的界值点
2.3. Ⅱ~Ⅲ期DLBCL患者预后单因素分析结果
2.4. Ⅱ~Ⅲ期DLBCL患者预后多因素分析结果
-
PET/CT是CT的解剖形态成像与PET的功能代谢显像的结合,在淋巴瘤的诊断、分期、疗效评价及预后评估中的作用明显优于CT检查,其常用的显像剂为18F-FDG,肿瘤细胞葡萄糖代谢异常活跃,相应地肿瘤病灶在18F-FDG PET/CT显像中为18F-FDG高摄取。有研究结果表明,肿瘤细胞恶性程度越高,细胞增殖越快,则摄取18F-FDG越高[6−7]。DLBCL是一类侵袭性淋巴瘤,对18F-FDG具有较高的亲和力,绝大多数DLBCL病灶的18F-FDG PET/CT显像为18F-FDG 高代谢。本研究85例DLBCL患者,18F-FDG PET/CT显像均表现为18F-FDG高代谢,SUVmax、MTV、TLG的中位数分别为23.9(16.3)、25.1(95.3)cm3、424.4(1404.6)。因此,18F-FDG PET/CT可作为DLBCL患者分期、疗效评价及预后判断的一种影像学检查技术。
DLBCL是一种异质性较高的肿瘤,近年来,尽管免疫化疗(利妥昔单抗+环磷酰胺+阿霉素+长春新碱+泼尼松方案)在临床治疗中的广泛应用,使DLBCL患者的生存期和生存率得到了明显改善,但是仍有部分患者在接受一线治疗后不能到达完全缓解或缓解后复发。目前,临床常用于判断DLBCL患者预后的评估指标为IPI,但是在利妥昔单抗时代,IPI的预后评价能力已在逐渐下降。因此,如何筛选出这些预后较差的高危患者,以便及早地进行干预和制定个体化治疗方案,提高临床治愈率及延长患者的生存期,已成为临床亟需解决的问题。
PET/CT自问世以来,PET代谢参数与DLBCL的预后关系一直是临床研究热点。SUVmax是临床常用的18F-FDG PET/CT半定量指标,反映了肿瘤18F-FDG摄取最高部位的代谢活性,其在DLBCL中的预后判断价值目前存在一定的争议。既往一些研究结果表明,SUVmax与DLBCL患者的预后相关,且是影响DLBCL患者预后的独立危险因素[8-9]。而另一些研究结果表明,SUVmax与DLBCL的预后无关[10-11],我们认为SUVmax受多种因素的影响,如患者血糖水平、仪器的衰减校正等,且SUVmax不能反映肿瘤的整体负荷情况,而DLBCL常常多发,肿瘤负荷较大。因此,治疗前DLBCL病灶SUVmax的预后判断价值存在一定局限性。基于此,近年来,一些新的PET代谢参数,如MTV、TLG则逐渐应用于临床,MTV是SUV在某一给定范围内PET图像上全部像素的体积,是一种基于肿瘤负荷的代谢参数,TLG则是MTV与平均SUVmean的乘积,既包含了肿瘤的18F-FDG代谢活跃程度,又兼顾了肿瘤的代谢体积,更接近于PET成像原理和肿瘤负荷的概念。Zhou等[12]回顾性分析91例DLBCL(Ⅰ/Ⅱ期患者34例,Ⅲ/Ⅳ期患者57例)的临床资料,结果表明治疗前18F-FDG PET/CT显像所获得的TLG是影响患者预后的独立危险因素,而SUVmax、MTV均不是。而Adams等[13]研究纳入了73例Ⅰ~Ⅳ期的DLBCL患者,多因素生存分析结果显示治疗前18F-FDG PET/CT得出的SUVmax、MTV、TLG均与患者的预后无关。因此,MTV、TLG在DLBCL患者的预后价值目前也存在一定的争议。Zhou等[12]和Adams等[13]研究同时都纳入了Ⅰ期和Ⅳ期的DLBCL患者,Ⅳ期患者易侵犯骨髓,而当骨髓浸润在PET/CT图像上表现为弥漫性18F-FDG代谢增高时,ROI的勾画较困难,MTV不易测量,同样TLG也不易获得;且当DLBCL累及消化道时,PET/CT也较难与肠管的炎性病变和生理性摄取鉴别;而Ⅰ期DLBCL患者,由于病灶较局限,患者的预后本身就明显好于Ⅲ期、Ⅳ期的患者。因此,本研究为排除上述干扰因素,仅纳入了Ⅱ期和Ⅲ期的DLBCL患者,结果表明:单因素分析时SUVmax、MTV、TLG是患者PFS的影响因素,MTV、TLG是患者OS的影响因素,由于MTV与TLG呈高度相关,因此多因素生存分析时没有纳入MTV,结果表明TLG是影响患者PFS和OS的独立危险因素,TLG越大,患者的预后越差;而SUVmax不是患者预后的独立危险因素。目前也有研究者将治疗前DLBCL患者的MTV与化疗中期PET/CT的多维尔评分(Deauville score,DS)进行结合,将患者分为:低危组(MTV<400)、中危组(MTV≥400,且DS≤3分)和高危组(MTV≥400,且DS>3分),高危组患者的5年PFS率明显低于低危组(29.7% vs. 90.9%,P<0.05),结果表明,治疗前18F-FDG PET/CT得出的MTV与中期PET/CT的DS评分结果进行结合,对患者的预后具有更好的判断价值[6]。我们后续研究将收集更多的病例,结合中期PET/CT显像结果来判断PET/CT在Ⅱ~Ⅲ期DLBCL患者中的预后价值。
本研究也存在不足。①本研究为回顾性研究,样本量较小;②一些淋巴结炎性病灶18F-FDG PET/CT可表现为18F-FDG高代谢,因此可能会高估MTV;③MTV的测量采用的是相对阈值法,可能会低估SUVmax较高病灶的MTV。目前,一种新的研究手段——纹理分析,即通过对CT、PET及PET/CT图像上的像素空间分布、强度等影像学特征进行数学分析和运算,从而对肿瘤的纹理参数进行量化,以便对肿瘤的诊断、疗效评价及预后评估进行判断。一项针对肺癌的研究结果表明,18F-FDG PET/CT得出的SUVmax、MTV、TLG均与患者的预后无关,而纹理分析所获得的量化指标是影响患者预后的独立危险因素[14]。我们团队后续将行PET/CT纹理分析与DLBCL患者的预后的相关研究。
总之,治疗前18F-FDG PET/CT得出的TLG是Ⅱ~Ⅲ期DLBCL患者预后的独立影响因素,对患者的预后判断具有一定的指导依据。
利益冲突 本研究由署名作者按以下贡献声明独立开展,不涉及任何利益冲突。
作者贡献声明 丁重阳负责数据的获取、提供与分析及论文的起草;丁磊负责数据的采集;李天女负责研究过程的实施;唐立钧负责数据的统计分析;柳卫负责研究命题的提出、设计及最终版本的修订。








 DownLoad:
DownLoad: