-
脑卒中也称脑中风或脑血管意外, 是一种由脑血流循环障碍所引起的急性缺血或出血性脑病, 进而导致突发意识及运动机能障碍的综合征。脑卒中分为出血性和缺血性脑卒中, 两者最终因缺血缺氧而引起神经元直接损伤。N-甲基-D-天冬氨酸受体(N-methyl-D-asprtate receptor)MDAR在中枢神经系统具有广泛且极为重要的生理功能, 在许多脑损伤疾病的病理过程中起着关键的作用, 其介导的兴奋性神经毒机制被认为是脑卒中的主要发病机制之一。本文综述了NMDAR与脑卒中关系及其核素显像剂用于脑卒中的早期诊治研究的最新进展。
HTML
-
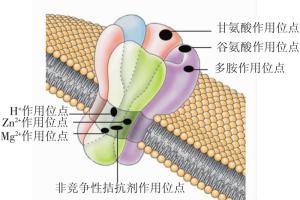
NMDAR作为主要的离子型谷氨酸受体, 是一种受配体和膜电位双重调节的配体门控离子通道, 其有多个特殊的结合位点, 如H+、Zn2+、Mg2+、非竞争性拮抗剂、谷氨酸、甘氨酸、多胺等结合位点(图 1)[1], 还可发生磷酸化等修饰性改变。NMDAR的激活具有特殊性, 对Ca2+的通透性比Na+或K+高[2]。NMDAR广泛分布于中枢神经系统, 但以海马和皮层最多, 其在突触传递及可塑性、学习和记忆等生理过程以及脑卒中兴奋性神经毒、神经退行性病变、精神疾病等病理过程中发挥着重要作用[3]。
NMDAR由NR1、NR2A、NR2B、NR2C、NR2D和NR3等亚基组成, NR1是功能亚基, NR2、NR3是调节亚基, 通常由2个NR1亚基和2个NR2亚基组成异四聚体[1, 4]。脑卒中缺血缺氧时, NR2A/NR2B比例表达下调, NMDAR功能发生改变[5-6], NR2B数目越多, 神经元对神经毒的易感性越强[7]。选择不同亚基作靶点, 可以研制有效的脑卒中治疗药物[8]。
-
脑卒中时, 缺血、缺氧将造成能量代谢障碍-兴奋性神经递质谷氨酸大量释放-谷氨酸受体(以NMDAR为主)过度激活-受体-效应器耦联异常-钙电导增加-神经元内钙超载-自由基反应-细胞死亡等, 这一系列缺血性连锁反应是导致脑卒中时脑损害的中心环节, 即缺血瀑布理论[9]。可见, 谷氨酸激活NMDAR引起的兴奋性神经毒效应在脑卒中的发生、发展中起关键作用, 脑卒中的缺血中心区即细胞水肿、坏死的梗死中心区, 缺血边缘区即半影区(或半暗带), 随着时间延长细胞可发生继发性死亡[10]。因此, 脑卒中的治疗有时间窗限制, 对脑卒中早期诊治能最大限度的挽救半影区神经细胞, 使脑功能受损程度减到最小[11]。
-
脑卒中时, 突触间隙增加的谷氨酸与突触后膜NMDAR结合, NMDAR过度激活, Ca2+通道打开, 胞内钙超载直接产生超氧阴离子(O2-), Ca2+与钙调蛋白结合, 一方面激活一氧化氮合酶(nitric oxide synthase, NOS), NOS催化L-精氨酸生成一氧化氮(nitric oxide, NO), NO又迅速与分子氧、超氧阴离子及铜、铁、镁等反应生成氧自由基产物, NO还与缺血条件下的大量超氧化物反应, 生成过氧亚硝酸阴离子(ONOO-)和羟自由基, ONOO-可造成DNA损伤和抑制线粒体的呼吸链功能, 产生ATP功能障碍, 促进氧化级联反应, 导致细胞的氧化损伤; 另一方面激活钙神经素, 使NOS去磷酸化而进一步激活NOS, 促进产生的NO-与O2-结合形成ONOO-, 加剧体内自由基大量生成。损伤的DNA激活聚腺苷二磷酸核糖聚合酶, 加剧能量耗竭, 最终导致神经元死亡[3]。Ca2+还可激活蛋白激酶、磷脂酶A2和磷脂酶C, 兴奋多价不饱和脂肪酸, 促使过氧化物和花生四烯酸生成增多, 钙泵活性减低, ATP产生不足, 反过来又促发突触末梢兴奋性氨基酸类神经递质的大量释放, 激活突触后的NMDAR, 细胞内Ca2+浓度进一步持续升高, 导致神经细胞损伤甚至死亡[12]。同时, 在缺血、缺氧条件下, 蛋白激酶C活性增加, 促进NMDAR磷酸化水平, 增加Ca2+内流, 后者又促进磷酸化程度[13]。
-
脑卒中时, NMDAR被过度激活, 离子通道异常, Ca2+、Na+和Cl-大量流入细胞内, K+外流, 使细胞内外离子失去平衡, 导致细胞水肿和神经传导异常, 而细胞内Ca2+释放的增加, 加剧了胞内钙的超载[14]。胞内钙超载除有上述作用机制外, 还通过激活核酸内切酶和凋亡蛋白酶促进神经元凋亡; 通过改变线粒体功能, 导致渗透性水肿或线粒体膜电位去极化, 线粒体氧化磷酸化失耦联或释放某些凋亡调控蛋白, 最终引起细胞凋亡; 通过激活半胱氨酸蛋白酶, 降解细胞骨架蛋白, 破坏微管, 导致细胞死亡。利用Ca2+通道阻滞剂可以对脑卒中动物模型起到脑保护作用, 细胞内Ca2+螯合剂1, 2-双(O-氨基苯氧基)乙烷-N, N, N', N'-四乙酸也能减轻缺血后神经元损伤[3]。
-
通过形态、生化、细胞和分子生物学的研究证明, 脑卒中时神经元的死亡包括坏死和凋亡, 其中早期引起的急性期神经元死亡以坏死为主, 而晚期的继发性死亡或迟发型死亡则以凋亡为主。神经元凋亡是一个通过合成新的蛋白质来实现的主动过程, 其中参与的因子主要有capsase家族蛋白酶、Bcl-2家族蛋白、丝裂原激活的蛋白激酶家族酶蛋白、线粒体释放蛋白、细胞溶酶体释放的多种酶蛋白、细胞核因子-κB、细胞因子及抑癌基因p53等。例如, capsase家族蛋白酶激活, 进而特异性识别、水解半胱氨酸和天冬氨酸残基, 导致DNA断裂, 神经元死亡, 随着病情的发展, 免疫炎症可促进细胞凋亡[10]。
2.1. 谷氨酸的兴奋性神经毒机制
2.2. 离子通道异常与缺血性神经元死亡
2.3. 细胞凋亡
-
NMDAR在脑卒中的发生、发展中起着关键作用, 因此研制特异性的NMDAR显像剂, 利用PET或SPECT研究NMDAR分布、数量和功能等的微量变化, 可对脑卒中作出早期诊断。
要成为一种理想的NMDAR显像剂, 其标记前体的选择很重要, 基于NMDAR的结构, 目前对NMDAR显像剂标记前体的研究重点是通过分析NMDAR亚基作用位点来确定, ①NR2亚基N末端区域: 作用于该区域的前体药物主要有艾芬地尔(ifenprodil)及其衍生物(主要作用于NR2B)和Zn2+(主要作用于NR2A)[15]; ②通道区域, 作用于该区域的前体药物需在NMDAR激活后通道打开, 才能起作用, 为非竞争性拮抗剂, 以电压依赖的方式起作用, 其主要的代表性前体药物有苯环己哌啶(phencyclidine, PCP)、噻吩环己哌啶(thienylcyclohexylpiperidine)、氯胺酮、地卓西平(dizocilpine, MK-801)和金刚烷类的衍生物美金刚(memantine)等[4]。不同亚基在不同疾病中所起的作用不同, 如脑卒中时以NR2B为主, 药物可作用于NR1与NR2亚单位的一种或数种, 从而有综合疗效[16]。
-
常见的NMDAR的PCP位点PET和SPECT显像剂有18F-MK-801、11C-氯胺酮、N-(1-萘基)-N′-(3-125I-碘苯基)-N′-甲基胍(N-(1-naphthyl)-N′-(3-125I-iodophenyl)-N′-methylguanidine, 125I-CNS 1261)、11CN-(2-氯-5-硫代甲烯基)-N′-(3-甲氧基-苯基)-N′-甲基胍(11C-N-(2-chloro-5-thiomethylenyl)-N′-(3-methoxyphenyl)-N′-methylguanidine, 11C-GMOM)等[17]。
(1) MK-801。主要通过降低NMDAR, 对脑卒中有脑保护作用[18]。Blin等[19]用18F-methyl-MK-801对狒狒进行PET发现, 在缺血、缺氧条件下, 18F-methyl-MK-801在脑局部的药物动力学改变不明显, 加上体内特异性结合低, 使其体内应用受到限制。考虑其体内特异性结合效率, Wallace等[20]用3H-MK-801定量放射自显影评价重摄取来分析大鼠大脑中动脉阻塞, 结果: 静脉给予3H-MK-801后15min, 缺血皮层和纹状体对该显像剂的聚集低于对侧大脑半球, 而60 min后则出现相反的现象, 表明早期对3HMK-801的摄取由脑血流量决定, 3H-MK-801能否作为脑卒中显像剂有待于进一步研究。将123I-MK-801用于人脑出血NMDAR SPECT发现, 给予显像剂后60~120 min, 皮质区和出血邻近区显像剂滞留的增加与NMDAR的激活相一致; 尽管123I-MK-801有可能作为一种SPECT显像剂来评价脑卒中时NMDAR的激活水平, 但由于其高脂溶性和由此而致的高非特异性结合, 限制了其使用[21]。
(2) 氯胺酮。其结合于NMDAR的PCP位点, 脂溶性较高, 易透过血脑屏障发挥抗兴奋性氨基酸的毒性作用, 降低NMDA通道的开放频率及开放时间, 减少NMDAR介导的Ca2+内流, 降低细胞内Ca2+超载对神经递质释放的刺激, 抑制缺血所致的Na+, K+-ATP酶活性的降低, 抑制NOS的活性。从而加强缺血细胞内Ca2+浓度的调节和对递质的再摄取, 在脑卒中时应用对神经细胞产生保护作用[22]。用11C-氯胺酮PET发现, 其在脑内有较高摄取, 但在脑内代谢及清除过快, 缺乏特异性结合[17], 使其在脑卒中的应用受到限制。
(3) 125I-CNS1261。Owens等[23]发现, 脑缺血大鼠对125I-CNS 1261的摄取在NMDAR分布区域较正常鼠增加, 体外实验证明其对NMDAR的MK-801结合位点具有高亲和力, 平衡解离常数值为(4.21±0.40)×10-9mol, 其脂水分配系数的对数(logD7.4)值为2.13, 而125I-MK-801为3.30;125ICNS 1261在体内代谢快, 半衰期为(2.17±0.44) min, 在给药后120 min, 脑组织匀浆仍可测到其活性 > 95%。123I-CNS 1261的特异性结合较高, 提前给予MK-801后, 123I-CNS1261与NMDAR的结合明显降低。在生理情况下, 123I-CNS 1261呈可逆性结合, 分布总体积定量参数可信, 从大到小依次为丘脑、纹状体、皮层、白质[24]。123I-CNS 1261在临床脑卒中的应用需进一步研究。
(4) 11C-GMOM。其对NMDAR的PCP位点有很高的亲和力, 脂溶性适中, 在啮齿类动物中的特异性结合为50%[17]。Waterhouse等[25]合成11C-GMOM后分析表明, 其平衡解离常数值为(5.2±0.3)×10-9mol, 脂水分配系数的对数为2.34, 放化纯度高达(96.7±1.5)%, 特异性活性为(1.25±0.45)×3.7 PBq/ mol; 3H-MK801的体内特性结合百分率为15%, 而11C-GMOM达50%, 这可能与GMOM的低脂溶性有关; 11C-GMOM在狒狒体内的特异性结合较大鼠体内低, 这说明物种差异使11C-GMOM的特异性结合在啮齿类动物与灵长类有差别。该显像剂可衡量NMDAR激活时的变化, 进而通过改变谷氨酸等神经递质而提供药物治疗基础, 这与临床脑卒中的发病有密切关系, 有早期诊治潜能。
(5) 美金刚。其通过与NMDAR的PCP位点结合而阻断NMDAR通道, 从而阻断Ca2+内流引起的后续级联反应, 用于脑卒中后有较好的脑保护作用[26], 还能减少室周白质化继发的神经功能缺失[27]。与其他非竞争性拮抗剂相比, 美金刚与PCP结合位点的亲和力低, 可快速结合与解离, 避免完全、持续对NMDAR通道的阻断, 耐受性较好、毒性低、不良反应小[28]。目前, 美金刚胺多用18F、99Tcm标记后作为放射性显像剂研究, 但在脑卒中的应用较少。
总之, 作用于NMDAR的PCP位点显像剂能否应用于人体, 与其亲和力、脂溶性及通道的激活程度有关。目前, 试验阶段的一些显像剂因其能严重阻断通道而有许多不良反应, 而通道的阻断程度与脂溶性和酸度系数有关, 所以, 研发亲和力较低、亲水性较高的显像剂可从一定程度上减少不良反应。但是, 低亲和力又难以成为一种有效的显像剂, Waterhouse[17]认为, 脂溶性比正常稍低、脂水分配系数的对数值在0.5~2.0之间比较合适。
-
色氨酸通过犬尿氨酸途径合成犬尿喹啉酸, 后者是NMDAR拮抗剂, 其通过与甘氨酸竞争结合NMDAR上的氨基乙酰位点, 从而阻断因NMDAR过度激活所引起的神经损伤, 减少神经毒物质, 如喹啉酸、犬尿氨酸、3-羟基氨基苯甲酸、邻氨基苯甲酸等, 而3-羟基氨基苯甲酸、邻氨基苯甲酸的增加与梗死灶体积的扩大相一致, 所以能减小梗死灶的体积, 减轻炎性反应和氧化应激反应, 起到神经保护作用[29]。Waterhouse等[30]发现, 3-[2-[(甲氧苯胺基)羰基]乙烯基]-4, 6-二氯吲哚-2-羧酸(3-[2-[(3-methoxyphenylamino)carbonyl]ethenyl]-4, 6-dichloroinole-2-carboxylic acid, 3MPICA)作为结合NMDAR甘氨酸位点高亲和力配体, 平衡解离常数值为(4.8± 0.9)×10-9mol, 其显像剂11C-3MPICA在大鼠生物学分布实验显示, 血液中放化活性较高, 但血脑屏障通过率低, 全脑的放射性清除较快, 其平均摄取2 min后小脑和丘脑分布较多, 但海马和皮层分布较少, 特异性结合与非特异性结合之比较低, 使11C-3MPICA作为脑卒中PET显像剂的体内应用受到限制。
此外, 脑卒中的潜在NMDAR显像剂还有多胺位点拮抗剂和氧化还原位点调节剂等, 有待进一步研究。
3.1. PCP位点显像剂
3.2. 甘氨酸位点显像剂
-
随着对NMDAR及其与脑卒中关系研究的深入, 人们认识到脑卒中后的病情发展是多因素作用的演变, 在这个连锁反应中, 有潜在的干预环节, 在此基础上的相关神经保护剂是除改善血流外的脑卒中治疗核心。脑卒中的治疗受治疗时间窗的限制, 因此早期诊断并及时予以干预是脑卒中预后的关键。作用于NMDAR的神经保护剂有潜在的诊治功效, 在了解各亚单位结构功能的基础上, 研发并证明能特异性作用于特定亚单位的药物极为关键。NMDAR的功能受协同激动剂甘氨酸调节, 可通过激动甘氨酸转运体1来增加对甘氨酸的重摄取, 减少作用于突触后膜NMDAR的甘氨酸的量, 最终下调NMDAR的过度激活。一些药物可选择性作用于NMDAR的调节酶, 如作用于NR2B的钙调蛋白依赖性蛋白激酶II, 有可能成为潜在的药物作用靶点[31]。相对于其他脑受体显像剂, NMDAR显像剂的研究较少, NMDAR显像剂应用于脑卒中的研究则更少, 今后应在NMDAR显像剂研究的基础上, 结合脑卒中的发病机制, 改善NMDAR显像剂的脂溶性、亲和力及特异性结合与非特异性结合之比, 提高脑部摄取量等。








 DownLoad:
DownLoad: