-
胃癌是最常见的恶性肿瘤之一。2020年全球癌症统计数据显示,在185个国家或地区的36种癌症中,胃癌的发病率居第5位,病死率居第4位[1]。我国胃癌患者总体5年生存率不足50%[2],转移和术后复发是其预后差的主要原因,其中淋巴结转移是主要危险因素之一[3]。评估淋巴结转移的重要指标之一是淋巴结大小,但胃癌患者中超过60%的转移淋巴结小于8 mm[4],导致CT检出率较低。通过18F-FDG PET/CT显像中葡萄糖代谢情况和淋巴结短径综合评估淋巴结转移,从而提高术前淋巴结分期的准确性[5]。然而,在临床上综合评估为N0期的胃癌患者中,仍可发现11%~56%的患者术后存在淋巴结转移[6-7],即隐匿性淋巴结转移(occult lymph node metastasis,OLM)。存在OLM的患者更易出现肿瘤复发,预后较差[8]。因此, OLM的早期确诊对临床医师精准制定治疗方案及预后评估具有重要意义。18F-FDG PET/CT有多个异质性指数(heterogeneity index, HI),如变异系数[9]、线性回归斜率[10]等,有研究者发现HI对于多种恶性肿瘤的OLM具有预测价值[11-12]。目前采用HI预测胃癌患者的OLM尚无有效依据。胃腺癌是胃癌最常见的类型,本研究旨在探讨胃腺癌患者术前18F-FDG PET/CT显像中原发灶的肿瘤内代谢HI对胃癌OLM的预测价值。
-
回顾性分析2016年1月至2022年12月于郑州大学第一附属医院术前行18F-FDG PET/CT检查的79例胃腺癌患者的临床资料,其中男性62例、女性17例,年龄(63.8±9.0)岁。所有患者均于显像后1个月内行胃腺癌根治术,根据术后组织病理学检查结果分为OLM阳性组(n=39)和OLM阴性组(n=40)。比较2组患者的年龄、性别、肿瘤原发灶部位、分化程度、Lauren分型、病理T分期、糖类抗原199(carbohydrate antigen199,CA199)、癌胚抗原(carcinoembryonic antigen,CEA)等。纳入标准:(1)组织病理学检查结果为胃腺癌;(2)18F-FDG PET/CT检查后1个月内行胃腺癌根治术 。排除标准:(1)术前影像检查发现任何区域疑似转移的淋巴结(CT或MRI图像上淋巴结短径≥10 mm或18F-FDG PET/CT图像上SUVmax≥2.5);(2)伴有远处转移;(3)行任何抗肿瘤治疗;(4)伴有其他活动性恶性肿瘤;(5)因胃充盈差导致病灶观察不清;(6)无组织病理学检查结果或临床资料不完整。因本研究为回顾性研究,豁免签署患者知情同意书。本研究符合《赫尔辛基宣言》的原则。
-
使用德国Siemens公司的Biograph Truepoint 64型PET/CT仪进行扫描。18F-FDG由日本住友集团的HM-20医用回旋加速器生产自动化合成模块合成,放射化学纯度≥98%。所有患者行PET/CT检查前禁食6 h以上,空腹血糖水平≤11.1 mmol/L。按患者体质量静脉注射18F-FDG 3.70~5.55 MBq/kg,嘱患者于安静状态下休息60 min后行PET/CT扫描,扫描范围自颅顶至大腿中上段。头部CT扫描参数:管电压120 kV、管电流380 mA、层厚3 mm;体部CT扫描参数:管电压120 kV、管电流由设备根据患者的身高、体质量及扫描部位自动调整,0.8 s/周。随后于相同扫描范围行PET三维扫描,扫描参数:头部3 min/床位,体部2.5 min/床位,共采集4~6个床位。采用德国Siemens公司的Syngo True D软件对PET/CT图像进行融合重建,最终得到横断面PET/CT融合图像。
-
由2名5年以上工作经验的核医学科主治医师独立阅片并对图像进行参数评估,意见不一致时与高年资主任医师讨论后决定。以原发灶SUVmax=2.5为阈值手动勾画ROI体积(volume of interest,VOI)。SUV主要包括SUVmax、SUVmean和标准化摄取值峰值(peak of standard uptake value,SUVpeak)。肿瘤/肝脏比值(TLR)为原发灶SUVmax与肝脏SUVmean的比值。体积参数包括肿瘤代谢体积(metabolic tumor volume,MTV )和糖酵解总量(total lesion glycolysis,TLG)。计算2个HI:(1)HI-1即CV,为SUV的标准差(standard deviation,SD)与SUVmean的比值,即SD/SUVmean如文献[9, 13]所述;(2)HI-2即根据不同的SUV临界值(40%SUVmax、60%SUVmax和80%SUVmax)对MTV进行线性回归的斜率的绝对值,通过对既往文献提出的方法[10, 14]修改后得出。
-
应用IBM SPSS 27.0软件对数据进行统计学分析。符合正态分布的计量资料以
$ \bar{x} $ $\chi^2 $ -
由表1可知,OLM阳性组与OLM阴性组间性别、分化程度及病理T分期差异均有统计学意义(均P<0.05),年龄、肿瘤原发灶部位、分化程度、Lauren分型、CA199水平升高和CEA水平升高组间差异均无统计学意义(均P>0.05)。
组别 年龄
(岁,$ \stackrel{-}{x} $ 

性别(例,%) 肿瘤原发灶部位(例,%) 分化程度(例,%) 男 女 GEJ及
胃底部胃体及
大小弯胃窦及
幽门区中分化 中-低分化 低分化 OLM阳性组(n=39) 62.82±10.48 27(69.2) 12(30.8) 16(41.0) 11(28.2) 12(30.8) 8(20.5) 12(30.8) 19(48.7) OLM阴性组(n=40) 64.80±7.22 35(87.5) 5(12.5) 21(52.5) 9(22.5) 10(25.0) 17(42.5) 13(32.5) 10(25.0) 检验值 z=−0.196 x2=3.903 x2=1.045 x2=6.061 P值 0.844 0.048 0.593 0.048 组别 Lauren分型(例,%) 病理T分期(例,%) CA199水平升高(例,%) CEA水平升高(例,%) 肠型 弥漫型 混合型 T1~T2 T3~T4 OLM阳性组(n=39) 16(41.0) 11(28.2) 12(30.8) 5(12.8) 34(87.2) 5(13.5) 4(10.8) OLM阴性组(n=40) 24(60.0) 9(22.5) 7(17.5) 15(37.5) 25(62.5) 7(18.9) 4(10.8) 检验值 x2=3.104 x2=6.361 x2=0.398 0 P值 0.212 0.012 0.528 1.00 Table 1. Comparison of clinical characteristics between the positive and negative groups in patients with occult lymph node metastasis of gastric adenocarcinoma
-
由表2可知, OLM阳性组原发灶HI-2明显高于OLM阴性组,且差异有统计学意义(P<0.05);而OLM阴性组原发灶SUVmax、SUVmean 、HI-1均明显高于OLM阳性组,且差异均有统计学意义(均P<0.05)。
组别 SUVmax SUVpeak SUVmean TLR MTV(cm3) TLG(g) HI-1 HI-2 OLM阳性组(n=39) 5.59
(4.46,7.51)4.37
(3.23,5.38)3.33
(3.06,3.85)2.75
(2.20,3.70)11.94
(3.23,30.90)39.87
(9.48,113.88)0.23±0.12 4.98
(2.68,8.44)OLM阴性组(n=40) 6.91
(5.11,10.64)5.09
(3.90,7.21)3.65
(3.25,4.64)3.30
(2.42,5.36)12.32
(3.37,20.86)43.07
(11.69,90.10)0.29±0.14 2.61
(1.84,4.23)检验值 z=−2.000 z=−1.736 z=−2.001 z=−1.314 z=−0.074 z=−0.255 t=2.096 z=−3.178 P值 0.045 0.083 0.045 0.189 0.941 0.799 0.039 0.001 Table 2. Comparison of metabolic parameters of 18F-FDG PET/CT between positive and negative groups of patients with occult lymph node metastasis in patients with occult lymph node metastasis of gastric adenocarcinoma [M(Q1, Q3) or
$ \bar{x} $ -
由表3单因素Logistic回归模型分析结果可知,分化程度、病理T分期、HI-1和HI-2是OLM的危险因素;由表3多因素Logistic回归模型分析结果可知,病理T分期和HI-2是胃腺癌患者OLM的独立危险因素。
临床特征和代谢参数 单因素分析
OR(95%CI)P值 多因素分析
OR(95%CI)P值 分化程度 0.054 0.174 中分化 1.000 1.000 中-低分化 1.962(0.621~6.193) 0.251 1.667(0.432~6.435) 0.459 低分化 4.037(1.295~12.585) 0.016 3.467(0.918~13.096) 0.067 病理T分期 T1-T2 1.000 1.000 T3-T4 4.080(1.310~12.709) 0.015 4.780(1.238~18.458) 0.023 SUVmax 0.891(0.792~1.003) 0.055 SUVpeak 0.877(0.758~1.014) 0.076 SUVmean 0.602(0.361~1.005) 0.052 TLR 0.808(0.631~1.034) 0.090 MTV(cm3) 0.999(0.977~1.021) 0.901 TLG(g) 0.998(0.994~1.003) 0.430 HI-1 0.025(0.001~0.992) 0.045 0.537(0.007~39.527) 0.777 HI-2 >4.962 7.368(2.385~22.764) <0.001 6.893(1.922~24.718) 0.003 ≤4.962 1.000 1.000 注:FDG为氟脱氧葡萄糖;PET为正电子发射断层显像术;CT为计算机体层摄影术;SUVmax为最大标准化摄取值;SUVpeak为标准化摄取值峰值;SUVmean为平均标准化摄取值;TLR为原发灶SUVmax/肝脏SUVmean的比值;MTV为肿瘤代谢体积;TLG为糖酵解总量;HI为异质性指数;CI为置信区间 Table 3. Logistic univariate and multivariate regression analysis of clinical characteristics and 18F-FDG PET/CT metabolic parameters in patients with occult lymph node metastasis of gastric adenocarcinoma
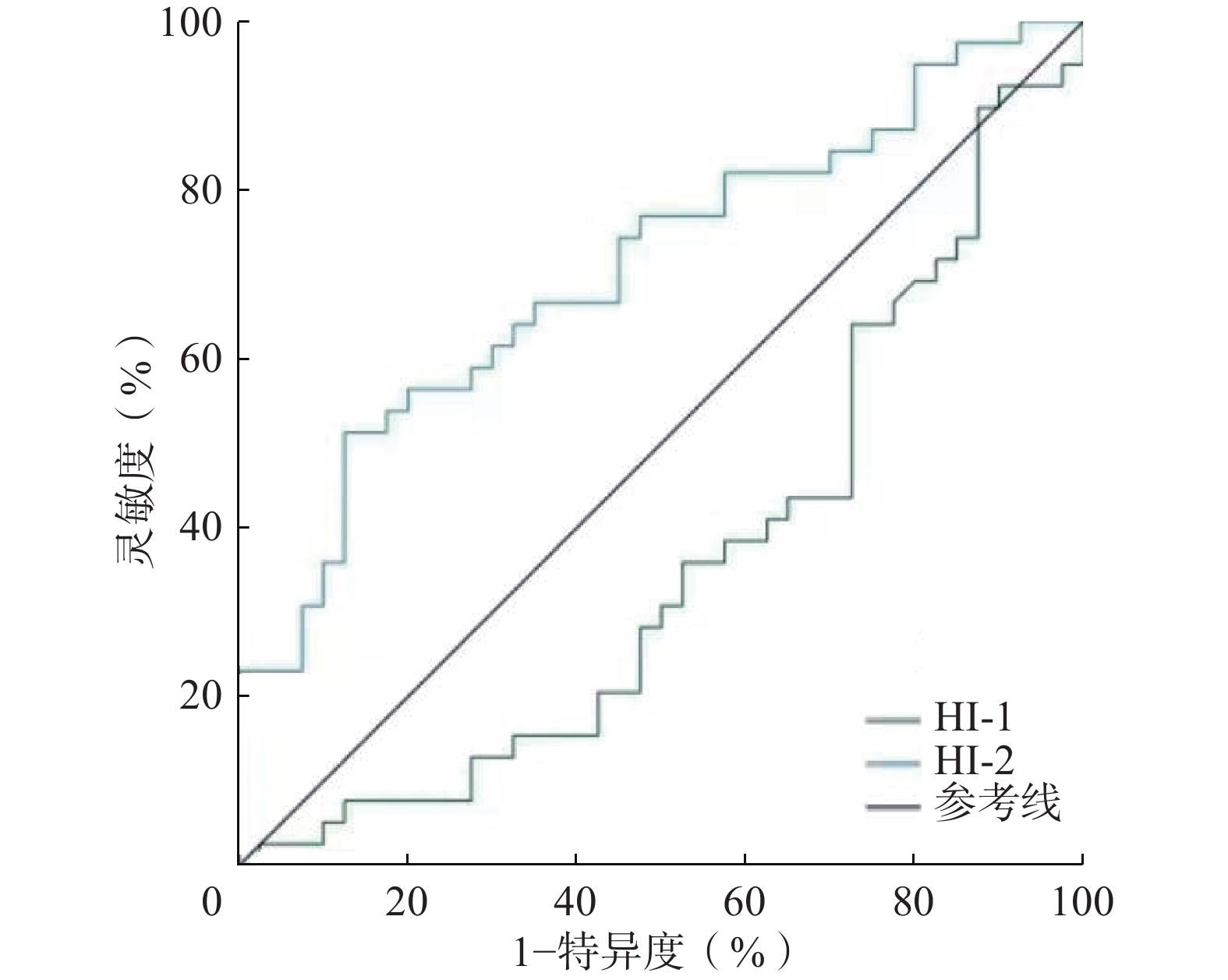
ROC曲线分析结果显示,HI-1预测OLM的AUC为0.360(95%CI:0.237~0.483,P=0.001),因此HI-1不具有诊断价值。HI-2预测OLM的AUC为0.708(95%CI:0.593~0.822,P<0.05)(图1),因此HI-2对OLM的诊断具有较好的准确率。当以最佳临界值4.962进行预测时,其诊断OLM的灵敏度和特异度分别为51.3%(20/39)和87.5%(35/40),典型患者图像见图2。
-
手术切除是治疗胃癌的主要方法。然而,大部分胃癌患者在手术切除后仍会复发。OLM是胃癌患者术后复发的主要危险因素。近年来,18F-FDG PET/CT的肿瘤内代谢HI在肿瘤的临床分期和预后预测方面具有重要价值[9,15-16]。Liu等[9]发现肿瘤原发灶的HI在评估胃癌患者预后中具有重要价值。Kim等[11]将HI用于食管癌区域淋巴结转移的预测,显示了其较好的预测价值。在本研究中,单因素及多因素Logistic回归模型分析的结果表明HI-2是胃癌OLM的独立预测因子。HI-2由不同SUV临界值下的系列MTV的线性回归斜率计算得出,在工作站上易获取,且重复性较高。18F-FDG PET/CT诊断胃癌淋巴结转移的特异性虽高达73%~92%,但灵敏度仅为40%~54.7%[17–19],在本研究中HI-2诊断OLM的灵敏度和特异度分别为51.3%和87.5%,因此肿瘤代谢HI对胃癌患者术前发现OLM具有重要的预测价值。
本研究发现肿瘤原发灶的SUVmax、SUVpeak、SUVmean及TLR在预测胃癌OLM方面无明显价值,这与Na等[20]的研究结果一致。但Yamada等[21]分析113例晚期胃癌患者的临床资料发现,肿瘤原发灶SUVmax是淋巴结转移的有效预测指标。张在炬等[22]发现SUVmax≥4.29作为胃癌淋巴结转移诊断标准时,其灵敏度和特异度分别为98.1%和45.7%。Oh[23]等发现SUVpeak可能是胃癌患者淋巴结转移的独立危险因素。造成以上研究结果差异的原因可能与肿瘤异质性、部分容积效应和样本差异有关[24]。本研究发现OLM阴性组SUVmax及SUVmean高于OLM阳性组,可能原因是阴性组中分化腺癌数目较多,这与既往研究结果[25-26]相符。
MTV和TLG是反映肿瘤负荷的代谢参数,但目前关于MTV和TLG在预测胃癌淋巴结转移的价值方面具有一定的争议。周锦等[27]发现MTV和TLG与肿瘤的N分期呈中度正相关。Xue等[28]发现MTV和TLG是胃癌淋巴结转移的独立预测因子。而王健林等[29]发现MTV和TLG与胃癌的N分期无明显相关性。本研究也未发现MTV和TLG在预测胃癌OLM方面的价值。上述研究结果存在差异的原因可能为:(1)MTV的获取方法尚未标准化,不同的SUV截断值结果不同;(2)本研究只分析了cN0期胃癌患者,与上述研究样本的范围不同;(3)胃部炎症及幽门螺杆菌感染等[30]均可导致对18F-FDG的高摄取,从而使代谢参数结果不可信。
我们的研究存在一定的局限性:(1)本研究是一项单中心、回顾性研究,纳入的患者数量相对较少;(2)MTV和HI的测量尚无标准化方法;(3)正常胃壁的生理性摄取可能会产生部分容积效应从而对肿瘤18F-FDG PET/CT的参数测量结果产生一定的影响,延迟显像或许可以降低胃壁生理性摄取的干扰,但需进一步的研究结果证实。
综上所述,肿瘤原发灶HI对胃癌OLM具有一定的预测价值,较高的HI-2是胃癌患者发生OLM的高危因素,这些发现有助于临床精准制定个体化治疗方案。
利益冲突 所有作者声明无利益冲突
作者贡献声明 王冉负责命题的提出与设计、数据的收集与分析、论文的撰写与修改;张涵玥负责文献的检索与整理;韩星敏负责论文最终版本的修订
Value of 18F-FDG PET/CT intra-tumor metabolic heterogeneity index for predicting occult lymph node metastasis in gastric adenocarcinoma
- Received Date: 2023-03-03
- Available Online: 2023-12-01
Abstract:









 DownLoad:
DownLoad: