-
非霍奇金淋巴瘤(non-Hodgkin's lymphoma,NHL)是一种起源于淋巴组织细胞,具有较强异质性的恶性肿瘤。NHL的治疗方法以综合治疗为主,如化疗、靶向治疗和免疫治疗。自体造血干细胞移植(autologous hematopoietic stem cell transplantation,ASCT)在NHL患者的治疗中也占据重要地位,美国国立综合癌症网络(National Comprehensive Cancer Network,NCCN)指南(第3版)指出,ASCT可作为一线诱导化疗后的巩固治疗应用于中-高危、高危NHL患者[1],同时,ASCT也适用于复发后对化疗敏感的NHL患者的挽救治疗[2-3]。NHL患者ASCT后的预后评估可以筛选出在ASCT后早期需要选择更适合的治疗方案(如放疗、放射免疫治疗、异基因干细胞移植等)的患者,使预后良好的患者避免接受过度治疗。
18F-FDG PET/CT在血液系统恶性肿瘤预后评估中的价值已经被证实,临床上已用于大多数淋巴瘤的分期、再分期和治疗反应的评估[4-7]。目前,18F-FDG PET/CT对淋巴瘤患者ASCT疗效评价的研究多根据Deauville评分(Deauvile score,DS)或Lugano标准进行[8-11]。2017 国际工作组共识认为淋巴瘤疗效评价标准(response evaluation criteria in lymphoma, RECIL)作为新的疗效评价标准正在临床逐渐得到应用[12]。RECIL基于18F-FDG PET/CT的代谢改变和CT的影像学改变,增加了轻微缓解(minor response,MiR)这一类别,对于疗效的评价更细化,有助于指导临床选择精准的治疗方案及在治疗过程中及时调整治疗方案。截至目前,国内鲜有研究分析RECIL在ASCT后NHL患者预后评估中的价值。本研究探讨了RECIL在NHL患者ASCT后预后评估中的价值,并与Lugano标准进行对比研究。
-
回顾性分析2010年10月至2021年11月于山西省肿瘤医院经组织病理学检查结果证实为NHL的86例初诊和(或)复发并行ASCT治疗患者的临床资料和影像资料,其中男性63例、女性23例,年龄34.0(22.0,47.0)岁。纳入标准:(1)组织病理学检查结果确诊为NHL,有详细且完整的临床资料;(2)ASCT前后均行18F-FDG PET/CT显像,显像分别在ASCT前2个月内和ASCT后1~6个月内进行;(3)均使用标准化疗方案进行治疗。排除标准:(1)患有中枢神经系统原发淋巴瘤;(2)伴发其他恶性肿瘤。86例患者中,弥漫大B细胞淋巴瘤(diffuse large B cell lymphoma,DLBCL)34例、自然杀伤/T细胞性淋巴瘤(natural killer/T-cell lymphoma,NKTCL)21例、其他类型淋巴瘤31例。本研究免除签署知情同意书。本研究获得山西省肿瘤医院伦理委员会的批准(批准号:KY2023015)。
收集所有患者ASCT治疗前后的18F-FDG PET/CT显像资料及完整的临床资料,包括性别、年龄、ASCT前病灶SUVmax、ASCT后病灶SUVmax、Ann Arbor分期、结外受累数目、组织病理学类型、东部肿瘤协作组体能状态(eastern cooperative oncology group,ECOG)评分、以年龄调整的国际预后指数(age-adjusted internationl prognostic indicator,aaIPI)评分、ASCT前化疗方案数、ASCT时机,并分别对患者进行危险度分层。所有患者均根据AnnArbor分期确定初诊时或挽救治疗前的分期,根据ASCT时机将患者分为一线巩固治疗组和挽救治疗组,在ASCT后根据RECIL对所有患者进行疗效评价,依据患者疗效评价结果将患者分为有效组:完全缓解(complete remisson,CR)、部分缓解(partial remission,PR)、MiR;无效组:疾病稳定(stable disease,SD)、疾病进展(progressive disease,PD)。
-
使用美国GE公司Discovery STE16 PET/CT仪进行显像,18F由美国GE公司MINItrace型回旋加速器生产,18F-FDG由化学合成模块自动合成,放射化学纯度>95%。嘱患者注射18F-FDG前24 h避免剧烈运动,并禁食6 h以上,血糖水平控制在<11.0 mmol/L,测量患者的身高和体重。患者在平静状态下经手背静脉或肘静脉注射18F-FDG ,剂量为4.44~5.55 MBq/kg,患者安静休息约1 h后排空膀胱并饮水约500 ml后行全身显像,显像范围自颅顶至股骨上段。先行CT扫描,扫描参数:管电压120 kV、管电流180 mA、螺距0.938、层厚3.75 mm、球管单圈旋转时间0.8 s。随后行PET扫描,共采集6~8个床位,采集时间3 min/床位。采用CT数据对PET图像进行衰减校正,用有序子集最大期望值迭代法重建PET图像,将PET和CT图像传送至美国GE公司AW 4.4工作站进行图像融合。
-
所有图像分别由2名具有5年以上诊断经验的核医学科医师独立阅片,观察病灶的大小、形态及18F-FDG的摄取情况,意见不一致时讨论达成一致。
DS疗效评价标准:1分为病灶对18F-FDG的摄取≤本底;2分为病灶对18F-FDG的摄取≤纵隔血池对18F-FDG的摄取;3分为纵隔血池对18F-FDG的摄取<病灶对18F-FDG的摄取≤肝血池对18F-FDG的摄取;4分为病灶对18F-FDG的摄取轻度高于肝血池对18F-FDG的摄取;5分为病灶对18F-FDG的摄取明显高于肝血池对18F-FDG的摄取(2~3倍以上);X为出现新病灶,但不太可能与淋巴瘤有关。
RECIL疗效评价标准:基于CT图像选取1~3个病灶,比较NHL患者ASCT后较ASCT前病灶长径总和(sum of longest diameters,SLD)的变化率。CR为DS 1~3分,且SLD下降≥30%;PR为DS 4~5分,且SLD下降≥30%;MiR为DS 1~5分,10%≤SLD下降<30%;SD为DS 1~5分,SLD下降<10%或SLD增加≤20%;PD为DS 1~5分,SLD增加>20%,或出现新的18F-FDG摄取增高病灶。
Lugano疗效评价标准:CR为DS 1~3分,ASCT后较ASCT前伴或不伴残余病灶;PR为DS 4~5分, ASCT后病灶18F-FDG的摄取较ASCT前减低;SD为DS 4~5分,ASCT后病灶18F-FDG的摄取较ASCT前无变化;PD为DS 4~5分,且ASCT后病灶18F-FDG的摄取较ASCT前增加和(或)出现新的淋巴瘤摄取病灶。
-
采用调阅电子病历和电话调查的方式对患者进行随访,随访截止时间为2022年11月1日,随访时间36(6~118)个月。总生存(overall survival,OS)期定义为从患者接受自体造血干细胞回输之日开始至任何原因的死亡或随访终止的间隔时间。OS率定义为在一定时间内患者未发生死亡的概率。
-
应用IBM SPSS 27.0软件对数据进行统计学分析。不符合正态分布的计量资料采用M(Q1,Q2)表示,计数资料以例数或百分比(%)表示。有效组和无效组患者18F-FDG PET/CT参数及临床特征的比较采用χ2检验或Mann-Whitney U检验;采用单因素及多因素Cox比例风险回归分析筛选影响ASCT后NHL患者预后的相关因素;采用Kappa检验评价RECIL和Lugano标准评估NHL患者ASCT后疗效的一致性(Kappa≥0.75为一致性较好,0.4<Kappa<0.75为一致性中等,Kappa≤0.4为一致性较差);采用Kaplan-Meier(K-M)生存分析比较RECIL与Lugano标准完全缓解组、部分缓解组和无效组3年OS率的差异;采用Log-rank检验分析3组间3年OS率的差异;采用ROC曲线分析RECIL和Lugano标准对NHL患者ASCT后3年OS率的预测效能。P<0.05(双侧)为差异有统计学意义。
-
86例NHL患者临床资料的比较见表1。有效组与无效组患者ASCT后病灶SUVmax、移植前化疗方案数和移植时机的差异均有统计学意义(均P<0.05),性别、年龄、移植前病灶SUVmax、Ann Arbor分期、结外受累数目、组织病理学类型、ECOG 评分和aaIPI评分的差异均无统计学意义(均P>0.05)(表1)。
项目 有效组(n=67) 无效组(n=19) 检验值 P值 性别(例,%) χ2=0.403 0.525 男 48(71.6) 15(78.9) 女 19(28.4) 4(21.1) 年龄(例,%) χ2=0.100 0.752 ≤35岁 38(56.7) 10(52.6) >35岁 29(43.3) 9(47.4) 移植前病灶SUVmax[M(Q1,Q2)] 1.6(1.1,2.2) 1.3(1.0,1.5) Z=−1.702 0.089 移植后病灶SUVmax[M(Q1,Q2)] 1.3(1.0,2.0) 5.2(4.8,8.9) Z=−6.149 <0.001 Ann Arbor分期(例,%) χ2=1.780 0.182 Ⅰ~Ⅱ 21(31.3) 3(15.8) Ⅲ~Ⅳ 46(68.7) 16(84.2) 结外受累数目(例,%) χ2=0.304 0.581 ≥2 27(40.3) 9(47.4) <2 40(59.7) 10(52.6) 组织病理学类型(例,%) χ2=4.035 0.127 DLBCL 30(44.8) 4(21.1) NKTCL 16(23.9) 5(26.3) 其他类型 21(31.3) 10(52.6) ECOG 评分(例,%) χ2=2.054 0.152 <2分 41(61.2) 15(78.9) ≥2分 26(38.8) 4(21.1) aaIPI评分(例,%) χ2=0.367 0.545 <2分 30(44.8) 10(52.6) ≥2分 37(55.2) 9(47.4) 移植前化疗方案数(例,%) χ2=11.949 <0.001 <2 44(65.7) 4(21.1) ≥2 23(34.3) 15(78.9) 移植时机(例,%) χ2=19.897 <0.001 挽救治疗组 11(16.4) 13(68.4) 一线巩固治疗组 56(83.6) 6(31.6) 注:FDG为氟脱氧葡萄糖;PET为正电子发射断层显像术;CT为计算机体层摄影术;RECIL为淋巴瘤疗效评价标准;SUVmax为最大标准化摄取值;DLBCL为弥漫大B细胞淋巴瘤;NKTCL为自然杀伤/T细胞性淋巴瘤;ECOG为东部肿瘤协作组体能状态;aaIPI为以年龄调整的国际预后指数 Table 1. The relationship between clinical data,18F-FDG PET/CT parameters of 86 patients with non-Hodgkin's lymphoma after autologous hematopoietic stem cell transplantation and the evaluation of response evaluation criteria in lymphoma efficacy
-
单因素Cox比例风险回归分析结果表明,移植后SUVmax、RECIL、移植前化疗方案数和移植时机是ASCT后NHL患者预后的影响因素(均P<0.05)(表2)。将RECIL、移植后SUVmax、移植前化疗方案数和移植时机纳入多因素Cox比例风险回归分析,结果显示RECIL是影响ASCT后NHL患者预后的独立危险因素(P<0.05)(表3)。
因素 HR值 95%CI P值 性别 1.587 0.343~7.350 0.555 年龄 1.035 0.991~1.080 0.119 移植前SUVmax 0.896 0.466~1.722 0.741 移植后SUVmax 1.177 1.087~1.274 <0.001 Ann Arbor分期 0.227 0.029~1.775 0.158 组织病理学类型 1.544 0.764~3.121 0.226 结外受累数目 1.158 0.339~3.962 0.815 ECOG 评分 5.932 0.759~46.377 0.090 aaIPI 评分 1.969 0.576~6.731 0.280 RECIL 0.020 0.003~0.155 <0.001 移植前化疗方案数 6.197 1.338~28.711 0.020 移植时机 8.808 2.289~33.891 0.002 注:FDG为氟脱氧葡萄糖;PET为正电子发射断层显像术;CT为计算机体层摄影术;RECIL为淋巴瘤疗效评价标准;SUVmax为最大标准化摄取值;ECOG为东部肿瘤协作组体能状态;aaIPI为以年龄调整的国际预后指数;HR为风险比;CI为置信区间 Table 2. Univariate Cox proportional risk regression analysis of the relationship between clinical features, 18F-FDG PET/CT parameters, response evaluation criteria in lymphoma and 3-year overall survival rate in patients with non-Hodgkin's lymphoma undergoing autologous hematopoietic stem cell transplantation
因素 HR值 95%CI P值 RECIL 0.040 0.004~0.439 0.008 移植后SUVmax 1.039 0.877~1.229 0.660 移植前化疗方案数 2.108 0.307~14.477 0.448 移植时机 1.756 0.382~8.081 0.469 注:FDG为氟脱氧葡萄糖;PET为正电子发射断层显像术;CT为计算机体层摄影术;RECIL为淋巴瘤疗效评价标准;SUVmax为最大标准化摄取值;HR为风险比;CI为置信区间 Table 3. Multivariate Cox proportional risk regression analysis of the relationship between clinical features, 18F-FDG PET/CT parameters, response evaluation criteria in lymphoma and 3-year overall survival rate in patients with non-Hodgkin's lymphoma undergoing autologous hematopoietic stem cell transplantation
-
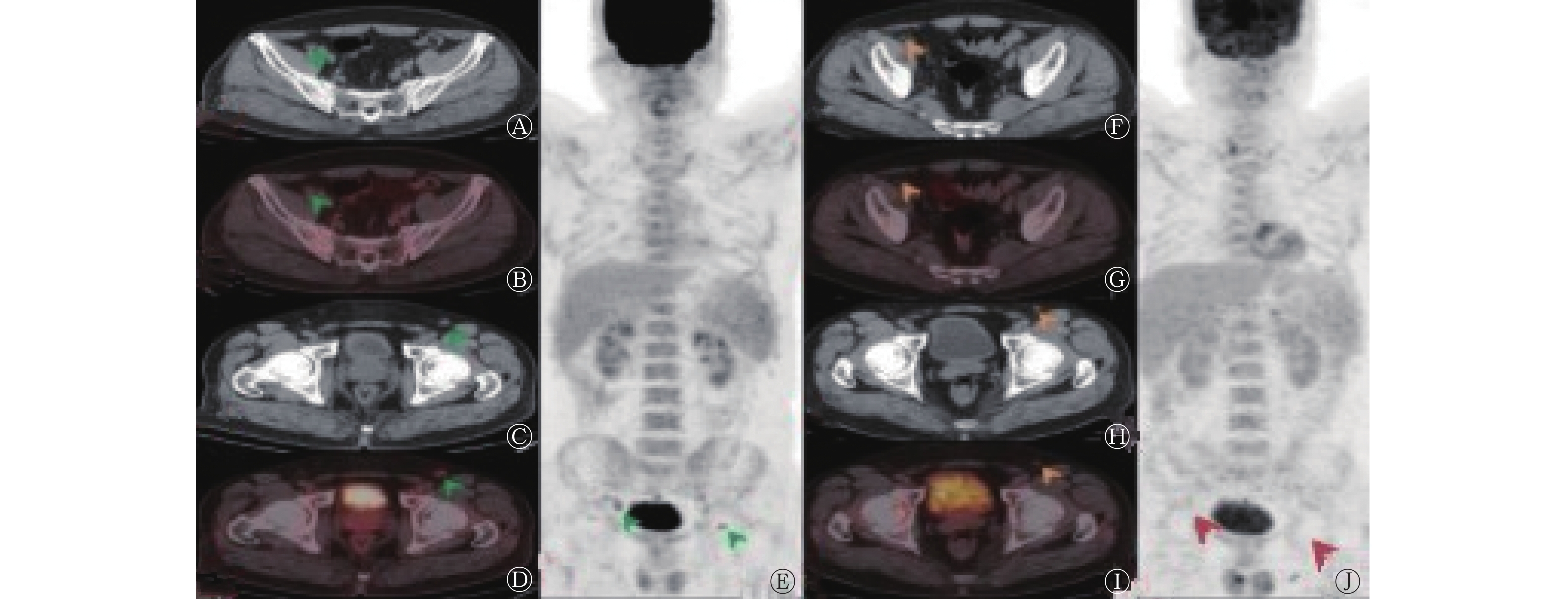
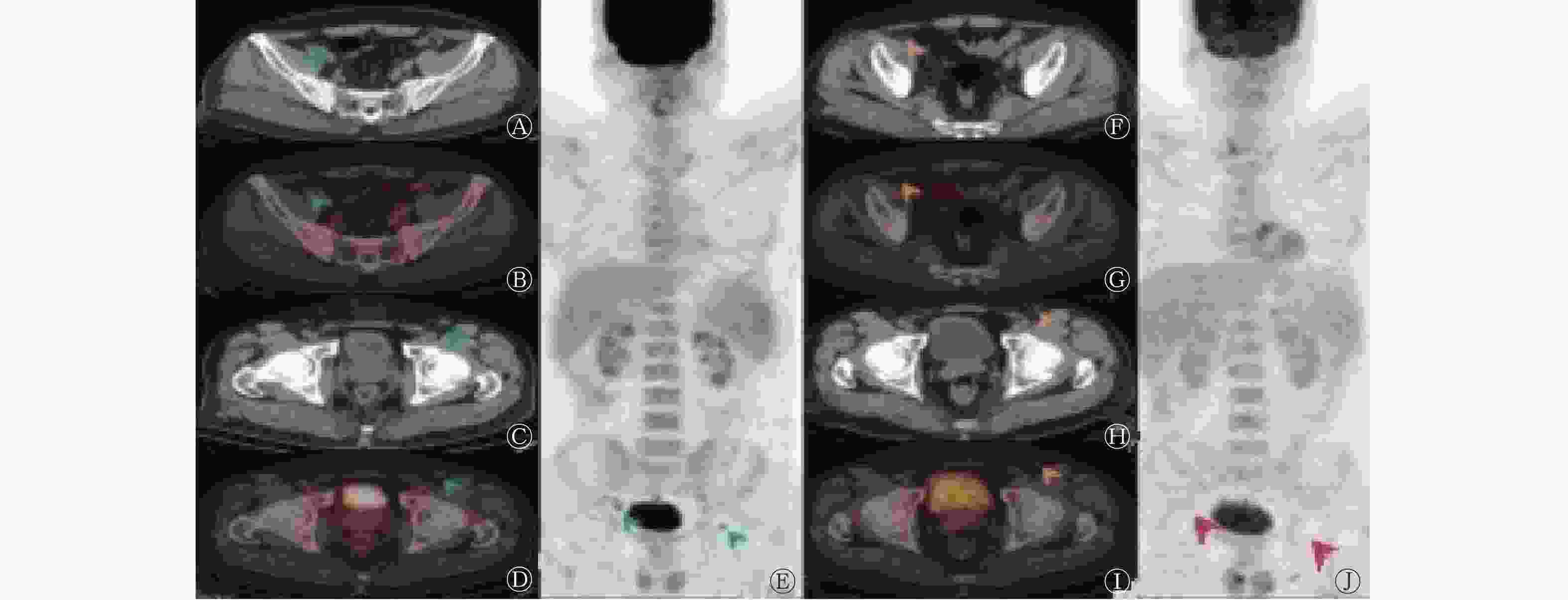
依据Lugano标准,CR患者 49例(57.0%,49/86)、PR 15例(17.4%,15/86)、SD 8例(9.3%,8/86)、PD 14例(16.3%,14/86);依据RECIL,将MiR患者纳入PR,则CR患者 49例(57.0%,49/86)、PR 18例(20.9%,18/86)、SD 2例(2.3%,2/86)、PD 17例(19.8%,17/86)。其中1例NKTCL患者(图1)依据RECIL评估为MiR,依据Lugano标准评估为CR。RECIL与Lugano标准对NHL患者ASCT后疗效评价的结果具有较好的一致性[86.0%(74/86),Kappa=0.77,P<0.001]。 -
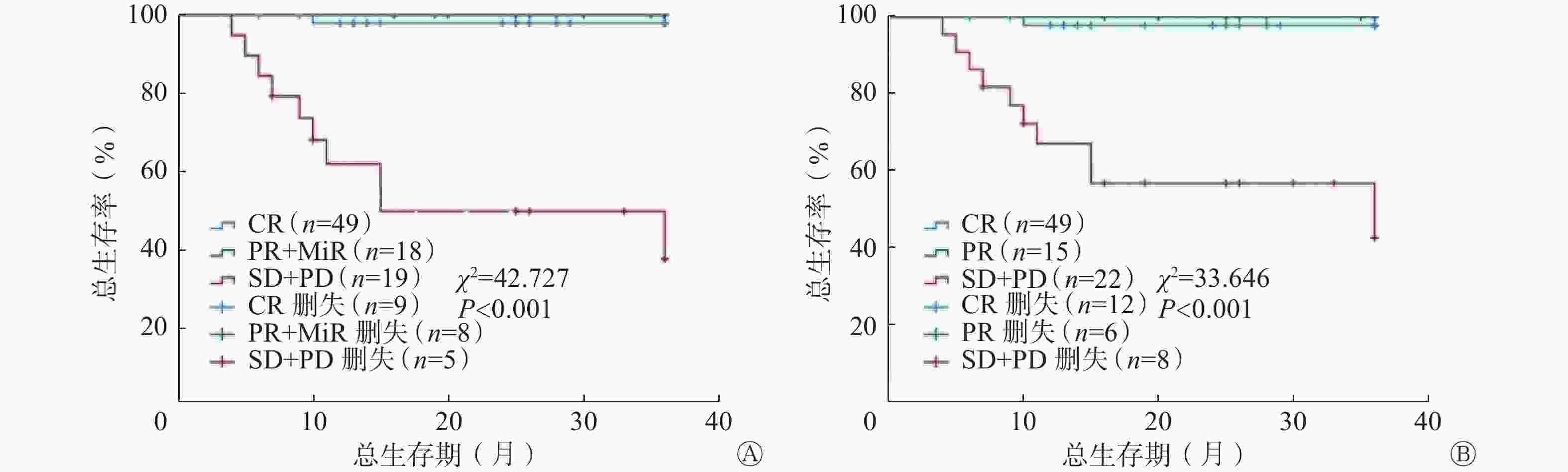
依据RECIL,将CR患者纳入完全缓解组,PR+MiR患者纳入部分缓解组,SD+PD患者纳入无效组。依据Lugano标准,将CR患者纳入完全缓解组,PR患者纳入部分缓解组,SD+PD患者纳入无效组。Kaplan-Meier生存分析结果显示,RECIL预测完全缓解组、部分缓解组、无效组患者3年OS率[2.0%(1/49) 对0(0/18)对52.6%(10/19)]的差异与Lugano标准预测3组患者3年OS率[2.0%(1/49)对0(0/15)对45.5%(10/22)]的差异均有统计学意义(χ2=42.727、33.646,均P<0.001)(图2)。
-
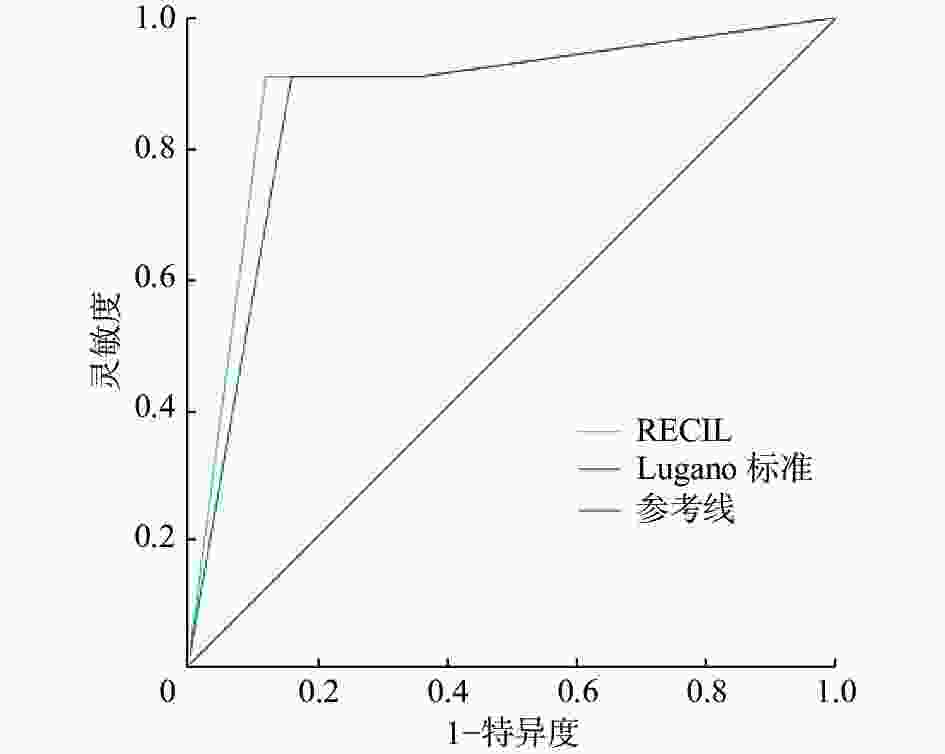
在预测预后的ROC曲线中,RECIL的AUC为0.884(95%CI:0.763~1.000,P<0.001),Lugano标准的AUC为0.865(95%CI:0.745~0.986,P<0.001);RECIL的AUC略高于Lugano标准,二者的差异无统计学意义(Z=1.334,P>0.05)(图3)。
-
目前,临床上用于淋巴瘤疗效评价的标准为Lugano标准,其基于PET/CT的代谢改变或CT的影像学改变对淋巴瘤的疗效进行评价;用于实体瘤的疗效评价标准为RECIST标准,其基于CT的影像学改变对淋巴瘤的疗效进行评价。为了使淋巴瘤与实体瘤的评价标准更好地统一,研究人员开发制定了RECIL,该标准基于18F-FDG PET/CT的代谢改变和CT的影像学改变[12]。Berzaczy等[13]研究发现,RECIL与Lugano标准对NHL患者化疗中期及化疗结束后的疗效评价具有较好的一致性,RECIL也可用于淋巴瘤患者疗效及预后反应的评估。Kostakoglu等[14]使用RECIL和Lugano标准对晚期滤泡性淋巴瘤(FL)患者进行的疗效评估结果也显示出高度一致性。Zheng等[15]的研究结果表明,RECIL可以预测行嵌合抗原受体T细胞(CAR-T)治疗的DLBCL患者的预后。本研究探讨RECIL对NHL患者ASCT后的预后评估价值并与Lugano标准进行对比研究。
国外研究结果显示,T细胞淋巴瘤患者ASCT后18F-FDG PET/CT显像阳性患者的预后较阴性患者差[16]。Stefani等[17]对47例接受ASCT治疗的NHL患者进行了回顾性研究,结果显示18F-FDG PET/CT可以很好地预测患者的预后。Armand等[18]的研究结果显示,18F-FDG PET/CT是评价ASCT后DLBCL患者预后的一个重要因素。国内亦有学者回顾性分析2007年6月至2016年5月行18F-FDG PET/CT显像的58例NHL患者的临床资料,结果显示ASCT后18F-FDG PET/CT显像阴性患者和阳性患者无进展生存(progression-free survival,PFS)率和OS率的差异均有统计学意义(均P<0.01)[19];Dai等[11]回顾性研究了2010年4月至2019年12月于ASCT后接受18F-FDG PET/CT显像的76例淋巴瘤患者的临床资料,结果显示ASCT后18F-FDG PET/CT显像阳性患者与较低的PFS率和OS率相关(均P<0.05)。另有研究结果表明,NHL患者ASCT后早期18F-FDG PET/CT显像Deauville标准对患者的PFS率和OS率皆无预测作用,这可能与纳入患者的组织病理学类型、18F-FDG PET/CT检查时间窗的设定及病例数有关[20]。本研究结果显示,ASCT后18F-FDG PET/CT可以评估NHL患者的预后,这与目前大多数研究的结果相似。
本研究中,单因素Cox比例风险回归分析结果显示,RECIL、移植后SUVmax、移植前化疗方案数及移植时机是NHL患者预后的影响因素。多因素Cox比例风险回归分析结果显示,RECIL是影响NHL患者预后的独立危险因素。Ying等[10]对79例接受ASCT治疗的NHL患者的18F-FDG PET/CT显像资料进行了回顾性分析,单因素分析结果显示,Deauville标准、移植前化疗方案数及移植时机是患者PFS率和OS率的影响因素;多因素分析结果显示,Deauville标准为患者PFS率和OS率的独立预后因素。已有研究结果证实,与挽救性ASCT相比,一线ASCT患者的OS率及PFS率均得到明显提高,一线ASCT可进一步提高高危患者的无病生存率[21]。本研究中,单因素Cox比例风险回归分析结果显示,移植时机是NHL患者预后的影响因素。
Berzaczy等[13]的研究结果表明,依据RECIL被评估为MiR的患者在化疗中期及化疗结束后的2年CR率均高于SD组患者(66.7% 对 25.0%,100% 对 0),因此本研究中依据RECIL对ASCT后NHL患者进行疗效评价时,根据评价结果将患者分为有效组和无效组,分别包括CR、PR、MiR,SD、PD,其中有效组患者67例,无效组19例。有效组和无效组患者ASCT后病灶SUVmax、移植前化疗方案数和移植时机的差异均有统计学意义。在与Lugano标准进行对比研究时,本研究将MiR患者纳入PR,结果表明,RECIL和Lugano标准对NHL患者ASCT后的疗效评价具有较好的一致性。Kaplan-Meier生存分析结果显示,2个标准均可用于NHL患者ASCT后的预后评估,RECIL的AUC略高于Lugano标准(0.884对0.865),2个标准预测预后的差异无统计学意义。
综上所述,RECIL与Lugano标准均可用于ASCT后NHL患者预后的评估。有研究结果显示,ASCT前行18F-FDG PET/CT显像可以预测淋巴瘤患者的预后[22]。由于ASCT患者初诊或复发时行18F-FDG PET/CT显像的患者有限,我们没有评估NHL患者ASCT前18F-FDG PET/CT显像RECIL与预后的关系,今后将增加样本量进行相关的研究。
利益冲突 所有作者声明无利益冲突
作者贡献声明 于青青负责数据的获取与分析、论文的撰写;赵铭负责研究命题的提出与设计、论文的修订;田蓉蓉、原凌、林艳梅负责研究过程的实施;王晋波、贾媛负责数据的统计与分析。
Prognostic evaluation of NHL patients after autologous hematopoietic stem cell transplantation using RECIL and comparative study with Lugano standard
- Received Date: 2023-01-16
- Available Online: 2023-11-25
Abstract:







 DownLoad:
DownLoad:

