-
甲状腺结节(thyroid nodule,TN)是指甲状腺细胞在甲状腺局部异常生长所引起的散在病变,临床常见,发病影响因素较多[1]。临床上,甲状腺炎症、甲状腺腺瘤和甲状腺囊肿等甲状腺疾病均可表现为TN。TN可为单发和多发,多发TN概率较高,但单发TN癌变风险较高[2]。TN多数为良性病变,少数为恶性病变。甲状腺癌前期无明显的临床特征,易漏诊,这使其发病风险在世界范围内显著增加[3],因此对TN的诊断及治疗至关重要。
目前,由于超声具有优异的时空分辨率,其已成为诊断和评估TN的首选检测技术,尤其是对于无症状的甲状腺癌[4]。随着临床上高频超声的广泛应用,TN的检出概率可达到68%[5]。评估TN的发生风险可帮助临床医师对其治疗方式进行选择。临床上采用甲状腺影像报告和数据系统(thyroid imaging reporting and data system,TIRADS)以提高诊断TN的准确率。2017年美国放射学会(American College of Radiology,ACR)提出的TIRADS(ACR-TIRADS)已在我国临床工作中广泛应用[6];同年,欧洲甲状腺协会也发布了成人TN超声恶性风险分层指南,即欧洲甲状腺协会甲状腺影像报告和数据系统(简称EU-TIRADS)[7]。2种TIRADS版本不同,其分层依据也不同,尚无统一标准[8]。目前,关于不同版本TIRADS的研究多数是对TIRADS诊断效能的研究,一致性研究较少,而可重复性和一致性对其是否能在临床上普及和推广十分重要。本研究就TN患者的ACR-TIRADS和EU-TIRADS资料进行综合评价,探究二者的诊断一致性及影响因素,从而为TN患者的诊断和预后以及TIRADS的临床适用性提供参考依据。
-
回顾性分析2019年6月至2022年1月于来安家宁医院(272例)和南京医科大学附属南京医院(10例)经细针穿刺活检(fine needle aspiration,FNA)或手术组织病理学检查结果确诊的282例TN患者的年龄、性别、TN情况(大小、个数、结构、回声、形状、边缘、钙化)、ACR-TIRADS和EU-TIRADS检查结果等资料,其中男性72例、女性210例,年龄(45.9±10.9)岁。纳入标准:(1)经FNA或手术获得组织病理学检查结果确诊;(2)临床资料完整。排除标准:(1)既往有甲状腺手术史;(2)肝肾功能不全、凝血功能障碍、意识障碍;(3)既往有颈部手术史。所有患者均于检查前签署了知情同意书。本研究通过了来安家宁医院医学伦理委员会的批准(批准号:20211208)。
-
使用美国GE公司的Logiq E9型彩色多普勒超声诊断仪(线阵探头,频率9~15 MHz)、佳能医疗系统有限公司的Aplio 700型彩色多普勒超声诊断仪(线阵探头,频率5~14 MHz)、日本东芝公司的Aplio 500型彩色多普勒超声诊断仪(线阵探头,探头频率5~11 MHz)对282例患者的所有TN分别进行ACR-TIRADS和EU-TIRADS分类。
由2位分别有12年和8年甲状腺超声诊断经验的医师进行标准化检查并存图。研究前系统学习ACR-TIRADS[9]和EU-TIRADS分类依据及相关超声征象并选取30个TN讨论并达成一致意见。意见不一致时,再取30个TN讨论直至意见一致。2位超声医师回顾入选患者超声图像,将入选患者的超声征象录入数据库。恶性TN超声诊断征象[10]:实性结节,低或极低回声,边界模糊、不规则,微钙化,纵横比值>1。
-
应用SPSS 23.0软件对数据进行统计学分析。符合正态分布的计量资料以
$\bar x\pm s $ 以FNA和手术获得的组织病理学检查结果为“金标准”,构建ACR-TIRADS和EU-TIRADS分类鉴别诊断良恶性TN的ROC,计算AUC,采用McNemar配对χ2检验比较二者的诊断准确性;采用多分类资料的Kappa检验进行一致性分析;计算灵敏度、特异度、准确率、阳性预测值、阴性预测值以及约登指数。采用Empower Stats和统计软件包“R”绘制森林图。采用Bootstrap法进行多因素Logistic回归预测模型校准度的内部验证。以ACR-TIRADS和EU-TIRADS检查结果相符为A组,不相符为B组,比较2组的超声诊断特征。以赋值为0的变量下的分层为对照分层,以赋值为1的变量下的分层为测试分层;以2种系统检查结果不相符为因变量,以A、B 2组患者超声诊断特征差异有统计学意义的指标为自变量,采用多因素Logistics回归预测模型分析ACR-TIRADS和EU-TIRADS检查结果不相符的独立危险因素。采用临床决策曲线评价模型的精准度。检验水准α=0.05。
-
282例患者共检测出320个TN,其中FNA检测出166个TN(118个良性TN、48个恶性TN);手术检测出154个TN(30个良性TN,124个恶性TN)。
-
由表1可知,TN的恶性风险均随着ACR-TIRADS和EU-TIRADS分类类别升高而增加。 ACR-TIRADS和EU-TIRADS构建ROC曲线的结果显示,其AUC分别为0.812(95%CI:0.771~0.853)和0.795(95%CI:0.754~0.836),约登指数最大值分别为0.544、0.531,所对应的临界值均为5类(图1)。
甲状腺结节 ACR-TIRADS[例(%)] EU-TIRADS[例(%)] 1类 2类 3类 4类 5类 2类 3类 4类 5类 良性结节(n=148) 4(2.70) 30(20.27) 29(19.59) 49(33.11) 36(24.32) 5(3.38) 55(37.16) 31(20.95) 57(38.51) 恶性结节(n=172) 0(0.00) 2(1.16) 3(1.74) 32(18.60) 135(78.49) 0(0.00) 6(3.49) 9(5.23) 157(91.28) 恶性风险(%) 0(0.00) 6.25 9.38 39.51 78.95 0(0.00) 9.84 22.50 73.36 注:数字1~5表示不同的分类类别。ACR-TIRADS为美国放射学会甲状腺影像报告和数据系统;EU-TIRADS为欧洲甲状腺协会甲状腺影像报告和数据系统 Table 1. Malignant risk analysis of thyroid nodule in American College of Radiology-thyroid imaging reporting and data system and European Thyroid Association-thyroid imaging reporting and data system
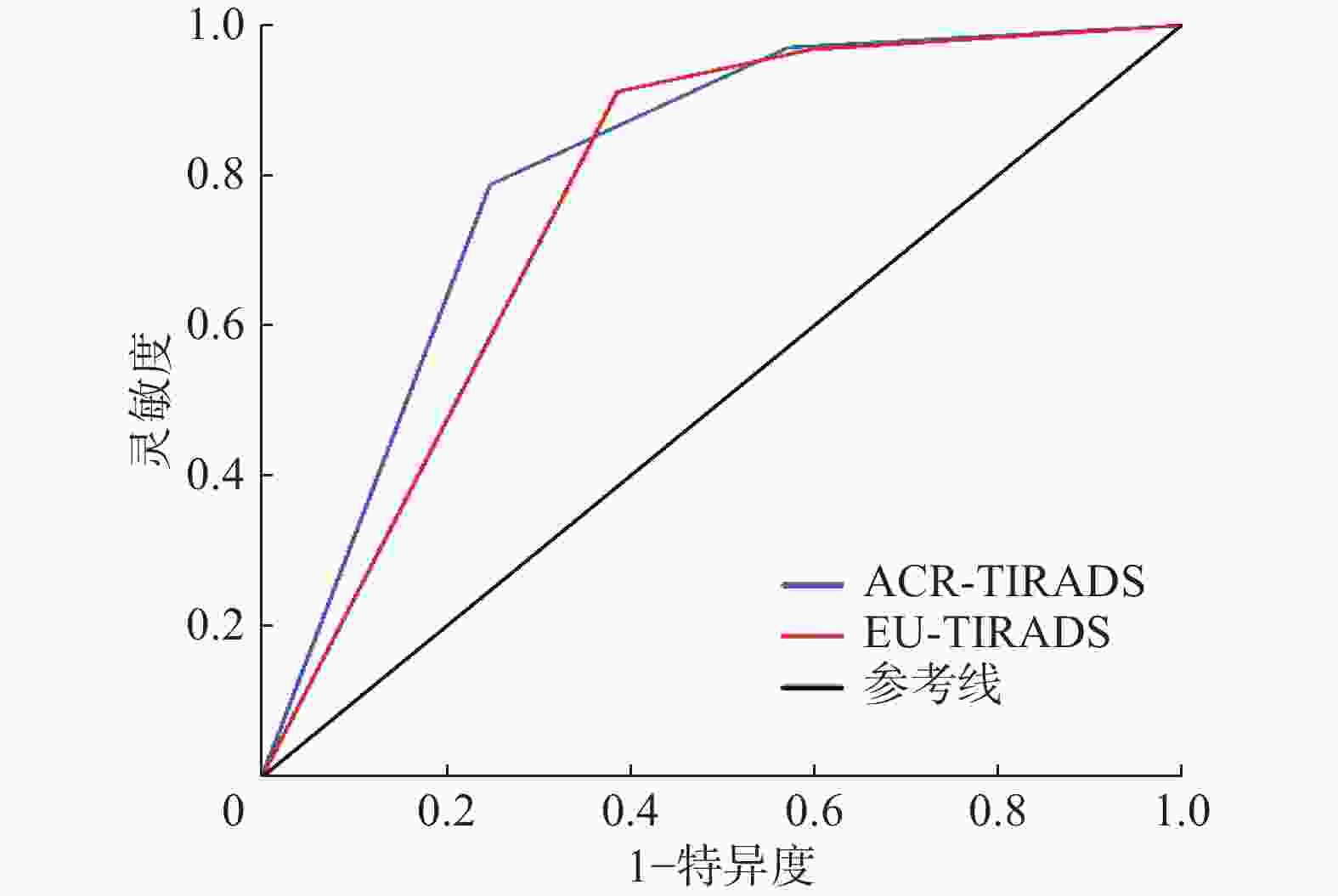
Figure 1. Receiver operating characteristic curves for differential diagnosis of benign and malignant thyroid nodules using American College of Radiology-thyroid imaging reporting and data system and European Thyroid Association-thyroid imaging reporting and data system
ACR-TIRADS和EU-TIRADS的诊断准确率(78.01%对77.62%)、阳性预测值(78.95%对73.36%)的差异均无统计学意义(χ2=0.036、2.796,P=0.849、0.095);EU-TIRADS较ACR-TIRADS的诊断阴性预测值(85.74%对74.75%)、灵敏度(91.54%对79.31%)均更高,差异均有统计学意义(χ2=12.034、19.135,P=0.001、<0.001);ACR-TIRADS较EU-TIRADS的诊断特异度(75.67%对62.38%)更高,差异有统计学意义(χ2=12.900,P<0.001)。
-
由表2、表3可知,ACR-TIRADS 2~4类无论是良性还是恶性TN均与EU-TIRADS 2~4类一致性良好。由表4可知,ACR-TIRADS和EU-TIRADS一致性分析结果显示,ACR-TIRADS 2类中所有TN(18个)均与EU-TIRADS 2类相对应;ACR-TIRADS 3类中有77个TN(96.25%,77/80)与EU-TIRADS 3类相对应;ACR-TIRADS 4类中有70个TN(94.59%,70/74)与EU-TIRADS 4类相对应;ACR-TIRADS 5类中有94个TN(63.51%,94/148)与EU-TIRADS 4类相对应,另外ACR-TIRADS 5类中有54个TN(36.49%,54/148)与EU-TIRADS 5类相对应。
ACR-TIRADS EU-TIRADS 合计 Kappa值 P值 准确率(%) 2 3 4 5 2 0(0.00) 0(0.00) 0(0.00) 0(0.00) 0(0.00) 0.375 <0.001 − 3 1(0.58) 8(4.65) 0(0.00) 0(0.00) 9(5.23) − − − 4 0(0.00) 0(0.00) 30(17.44) 0(0.00) 30(17.44) − − − 5 0(0.00) 0(0.00) 85(49.42) 48(27.91) 133(77.33) − − − 合计 1(0.58) 8(4.65) 115(66.86) 48(27.91) 172(100.00) − − 50.00 注:数字2~5表示不同的分类类别;−表示无此项数据。ACR-TIRADS为美国放射学会提出的甲状腺影像报告与数据系统;EU-TIRADS为欧洲甲状腺协会提出的甲状腺影像报告与数据系统 Table 2. Consistency analysis of American College of Radiology-thyroid imaging reporting and data system and European-thyroid imaging reporting and data system in the examination results of malignant thyroid nodule (cases (%))
ACR-TIRADS EU-TIRADS 合计 Kappa值 P值 准确率(%) 2 3 4 5 2 18(12.16) 0(0.00) 0(0.00) 0(0.00) 18(12.16) 0.844 <0.001 − 3 2(1.35) 69(46.62) 0(0.00) 0(0.00) 71(47.97) − − − 4 0(0.00) 2(1.35) 40(27.03) 2(1.35) 44(29.73) − − − 5 0(0.00) 0(0.00) 9(6.08) 6(4.05) 15(10.14) − − − 合计 20(13.51) 71(47.97) 49(33.11) 8(5.41) 148(100.00) − − 89.86 注:数字2~5表示不同的分类类别;−表示无此项数据。ACR-TIRADS为美国放射学会提出的甲状腺影像报告与数据系统;EU-TIRADS为欧洲甲状腺协会提出的甲状腺影像报告与数据系统 Table 3. Consistency analysis of American College of Radiology-thyroid imaging reporting and data system and European-thyroid imaging reporting and data system in the examination results of benign thyroid nodule (cases (%))
ACR-TIRADS EU-TIRADS 合计 Kappa值 P值 准确率(%) 2 3 4 5 2 18(5.63) 0(0.00) 0(0.00) 0(0.00) 18(5.63) 0.571 <0.001 − 3 3(0.94) 77(24.06) 0(0.00) 0(0.00) 80(25.00) − − − 4 0(0.00) 2(0.63) 70(21.88) 2(0.63) 74(23.13) − − − 5 0(0.00) 0(0.00) 94(29.38) 54(16.88) 148(46.25) − − − 合计 21(6.56) 79(24.69) 164(51.25) 56(17.50) 320(100.00) − − 68.44 注:数字2~5表示不同的分类类别;−表示无此项数据。ACR-TIRADS为美国放射学会提出的甲状腺影像报告与数据系统;EU-TIRADS为欧洲甲状腺协会提出的甲状腺影像报告与数据系统 Table 4. Consistency analyzing of American College of Radiology-thyroid imaging reporting and data system and European-thyroid imaging reporting and data system in the examination results of thyroid nodules (cases (%))
-
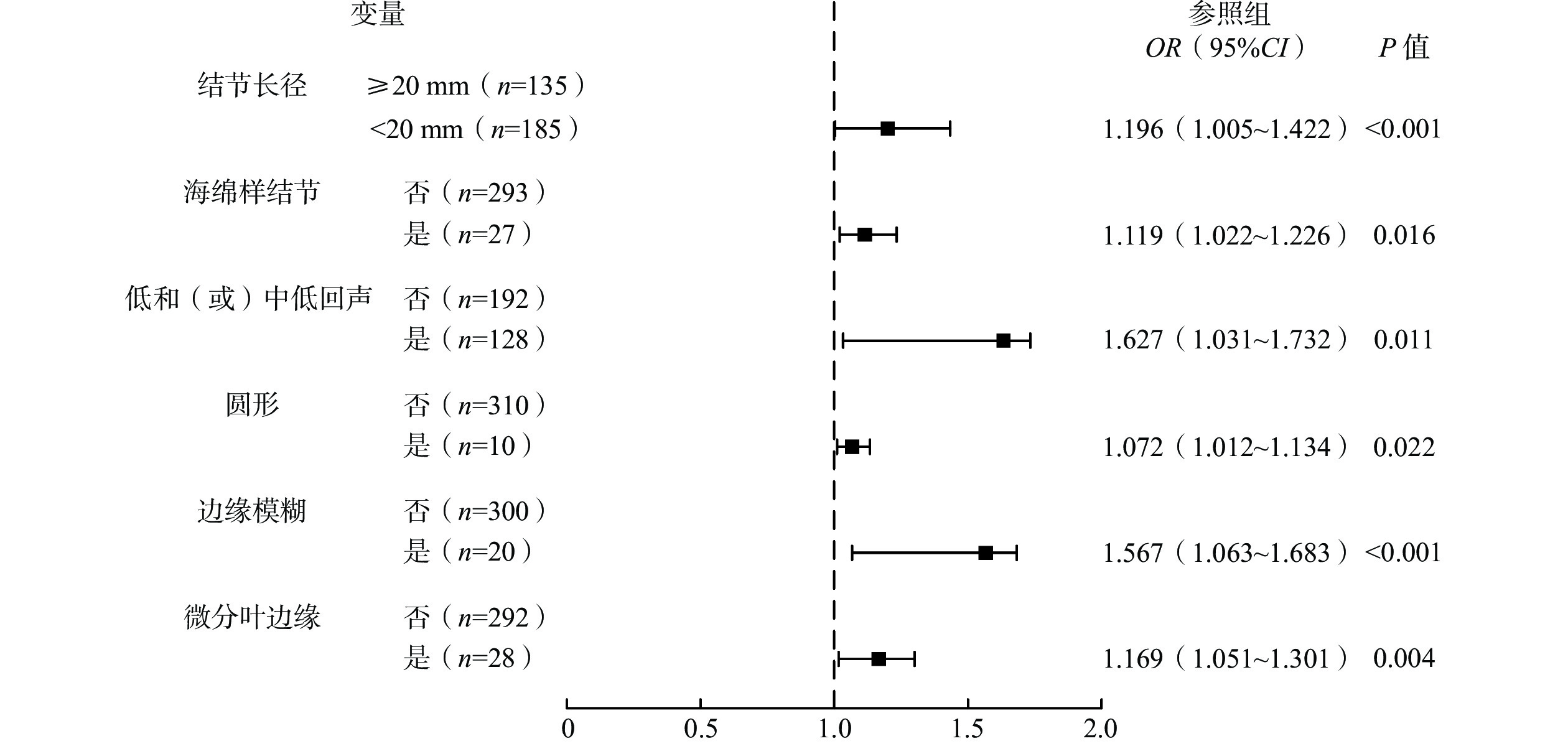
ACR-TIRADS和EU-TIRADS检查结果相符的A组TN共219个,不相符的B组TN共 101个。A、B 2组的超声诊断特征比较结果显示,结节长径(10~15 mm、≥20 mm)、囊性或几乎囊性、海绵样结节、低和(或)中低回声、圆形、边缘以及微钙化均是ACR-TIRADS和EU-TIRADS检查结果不相符的相关因素,差异均有统计学意义(均P<0.05,表5)。多因素Logistic回归分析结果显示,结节长径<20 mm、海绵样结节、低和(或)中低回声、圆形、边缘模糊以及微分叶边缘,均是2种系统检查结果不相符的独立危险因素(均P<0.05,图2)。
组别 结节长径 结构 5~10 mm 10~15 mm 15~20 mm ≥20 mm 囊性或几乎囊性 囊实性 实性或几乎实性 海绵样结节 A组(n=219) 25(11.42) 42(19.18) 39(17.81) 113(51.60) 19(8.68) 54(24.66) 136(62.10) 10(4.57) B组(n=101) 14(13.86) 42(41.58) 23(22.77) 22(21.78) 2(1.98) 21(20.79) 61(60.40) 17(16.83) χ2值 0.386 17.925 1.090 25.195 5.054 0.576 0.085 13.460 P值 0.534 <0.001 0.296 <0.001 0.025 0.448 0.771 <0.001 组别 回声 形状 边缘 高回声或等回声 低和(或)
中低回声极和(或)
显著低回声卵圆形 圆形 直立生长
(纵横比值>1)光滑 模糊 A组(n=219) 98(44.75) 77(35.16) 44(20.09) 175(79.91) 2(0.91) 42(19.18) 187(85.39) 7(3.20) B组(n=101) 35(34.65) 51(50.50) 15(14.85) 80(79.21) 8(7.92) 13(12.87) 53(52.48) 13(12.87) χ2值 2.900 6.773 1.262 0.021 11.212 1.932 39.934 11.042 P值 0.089 0.009 0.261 0.885 0.001 0.165 <0.001 0.001 组别 边缘 钙化 微分叶 针刺样边缘 甲状腺外侵犯 粗大钙化 边缘钙化 微钙化 孤立性性钙化 A组(n=219) 12(5.48) 11(5.02) 2(0.91) 12(5.48) 5(2.28) 62(28.31) 3(1.37) B组(n=101) 16(15.84) 14(13.86) 5(4.95) 8(7.92) 1(0.99) 17(16.83) 0(0.00) χ2值 9.296 7.497 5.266 0.703 0.628 4.899 1.397 P值 0.002 0.006 0.022 0.402 0.428 0.027 0.237 注:ACR-TIRADS为美国放射学会甲状腺影像报告和数据系统;EU-TIRADS为欧洲甲状腺协会甲状腺影像报告和数据系统 Table 5. Comparison of ultrasound diagnostic characteristics between two groups of patients with consistent or inconsistent American College of Radiology-thyroid imaging reporting and data system and European-thyroid imaging reporting and data system examination results (cases (%))
-
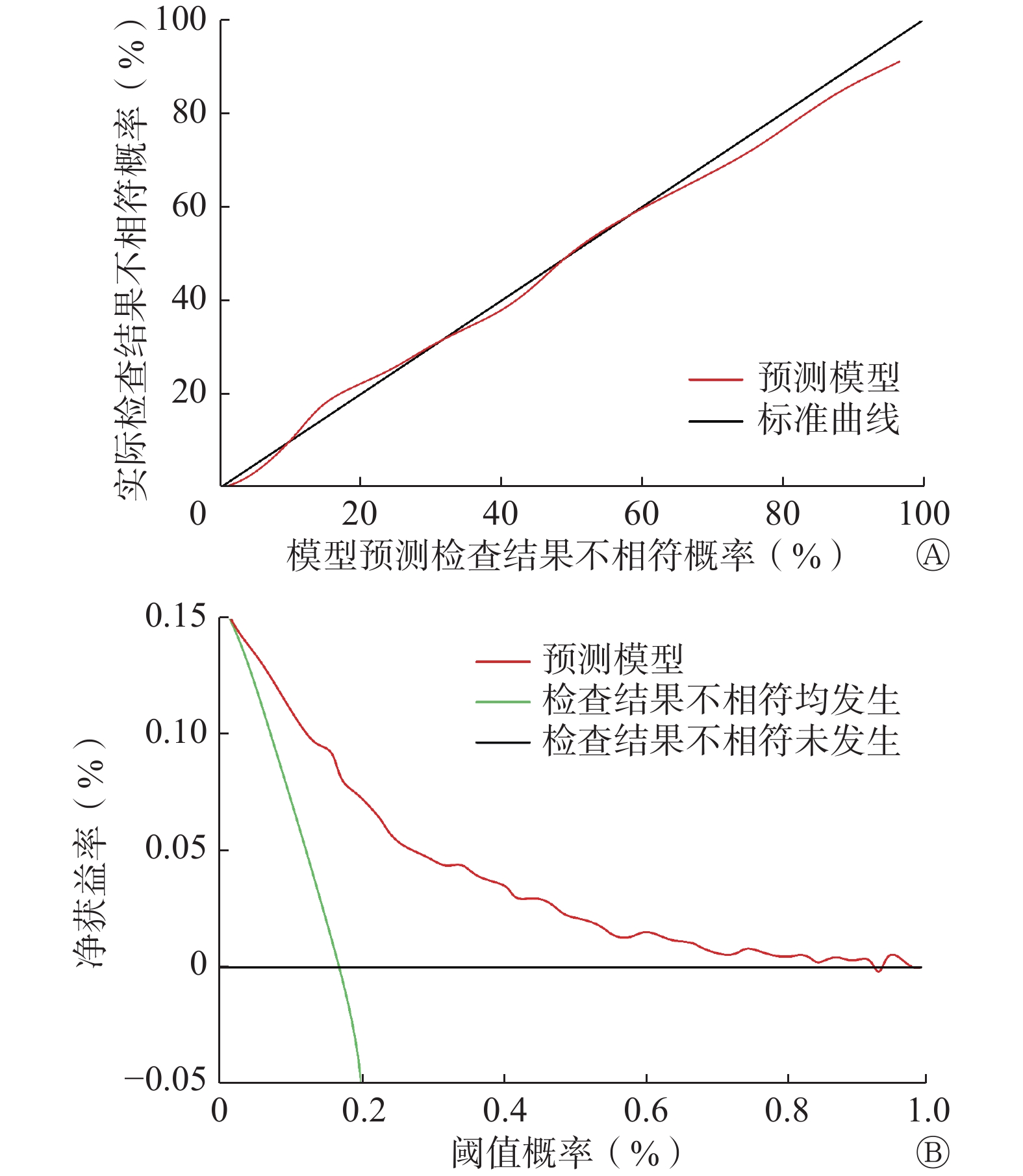
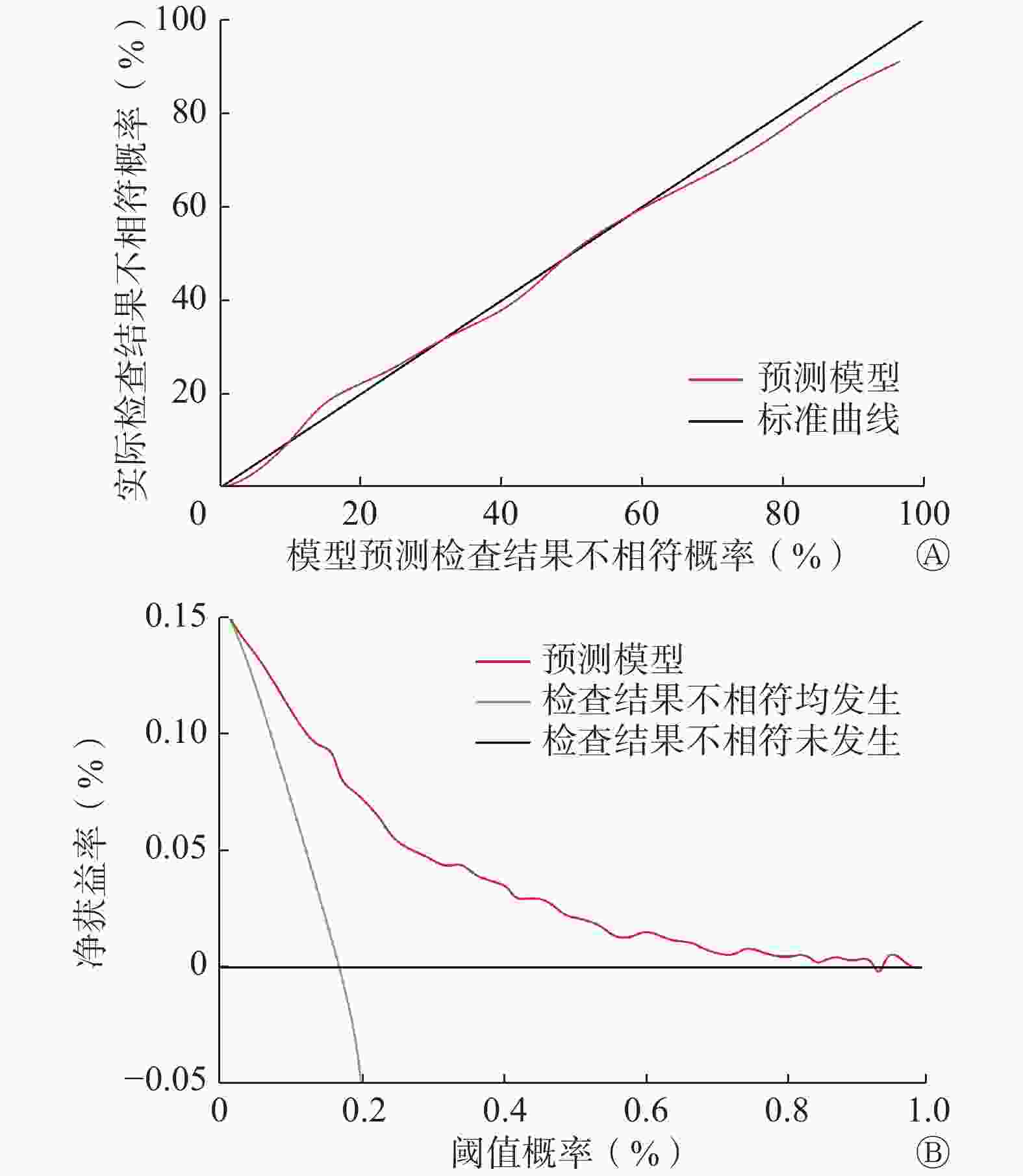
经Bootstrap法内部验证后C指数相同,多因素Logistic回归模型预测ACR-TIRADS和EU-TIRADS检查结果不相符情况与实际情况一致。校准图显示,Logistic回归预测模型与标准曲线拟合度良好(图3A)。通过临床决策曲线评估模型对预测ACR-TIRADS和EU-TIRADS检查结果不相符风险的临床净获益情况。模型阈值概率在0.01~0.94区间时,净获益率>0(图3B)。
-
据统计,在一般人群中TN的发病概率为4%~8%,且多数患者经手术组织病理学诊断后已是中晚期[11]。目前,TN的诊断方法有多种,包括触诊、超声影像学、FNA或手术组织病理学检查等。其中触诊虽简单、方便,但结果主观;FNA虽创伤小,特异度和灵敏度高,但穿刺失败风险较高,尤其是对于小或微小结节以及多发结节;而手术组织病理学是通过手术切除病变组织,风险较高,对机体功能损害大并有伴多种并发症的可能[12]。在我国TN的超声诊断方法中应用最广泛的是ACR-TIRADS,而EU-TIRADS较少,相关研究结果表明,这2种分类系统对良恶性TN的诊断效能均较高[13]。
在本研究中,TN恶性风险随着ACR-TIRADS和EU-TIRADS分类类别升高而增加。ACR-TIRADS 2~4类无论是良性还是恶性TN均与EU-TIRADS 2~4类一致性良好,而ACR-TIRADS 5类的TN中有63.51%(94/148)分布于EU-TIRADS 4类,有36.49%(54/148)分布于EU-TIRADS 5类。这可能是由于ACR-TIRADS采用得分制,以极低回声、高>宽、甲状腺外侵袭以及点状强回声的权重最大;而EU-TIRADS采用分层制,具备≥1个恶性特征的TN归入5类,导致二者5类恶性TN检查结果显著不一致。与ACR-TIRADS相比,EU-TIRADS的灵敏度(91.54%对 79.31%)较高、特异度(62.38%对75.67%)较低,这与Schenke等[14]和Shen等[15]的研究结果相符。
为进一步明确影响二者检查结果不相符的相关因素,本研究采用多因素Logistic回归预测模型进行分析,结果显示,结节长径<20 mm、海绵样结节、低和(或)中低回声、圆形、边缘模糊以及微分叶边缘,均是二者检查结果不相符的独立危险因素。原因可能为:(1)由于本研究中恶性TN占比较高53.75%(172/320),因此当结节长径≥20 mm时,超声表现出纵向生长及边缘侵犯等特征的概率较高,而2种系统对这些特征的灵敏度均较高,故二者一致性较好,具体情况还有待进一步研究。(2)海绵样结节是一种完全微囊结构,评估难度较大,需明确其是否为完全微囊结构,故二者一致性较差。(3)EU-TIRADS不会根据混合回声结节实性部分的主要回声进行判断,其中若有低回声,则判断为低和(或)中低回声,结节周边回声会影响回声判断,特别是在炎症反应背景下,故判断低和(或)中低回声具有一定主观性,以致二者检查结果不相符。(4)EU-TIRADS的分层依据为结节形状,对于低和(或)中危类,圆形无法归类,故二者一致性较差。(5)在等回声或不均匀回声背景下,如弥漫性甲状腺炎,判断边缘模糊的结节有较大主观性,导致二者检查结果不相符。(6)微分叶是指1个或以上光滑、圆形局部凸起,当凸起单一且不明显时,二者存在较大分歧。Logistic回归预测模型的评价结果显示,其准确度较高。
本研究为回顾性研究,存在一定的局限性,尽管规范检查且标准化采集图像,但在一定程度上,静态存图会影响各超声特征的评估,而实时动态评估更精确。
综上所述,EU-TIRADS较ACR-TIRADS对TN的灵敏度高、特异度低;二者对2~4类TN的检查结果一致性良好;结节长径<20 mm、海绵样结节、低和(或)中低回声、圆形、边缘模糊以及微分叶边缘,均是影响二者检查结果一致性的独立危险因素。
利益冲突 所有作者声明无利益冲突
作者贡献声明 贺然负责命题的提出、研究方法的设计、数据的统计与分析、论文的撰写;郝祥玉负责数据的获取、论文的审阅与修改;卢晓莉负责论文最终版本的修订
Analysis of consistency and difference between ACR-TIRADS and EU-TIRADS in the diagnosis of thyroid nodules
- Received Date: 2022-12-21
- Available Online: 2024-02-25
Abstract:








 DownLoad:
DownLoad: