-
Lung cancer is the most common male cancer with leading a leading death rate in China[1]. Except surgery, chemotherapy and biotherapy, radiotherapy is an effective strategy for therapy of lung cancer[2]. However, occurrence of resistance results in radiotherapy failure, but little is known about its underlying mechanisms. A growing number of studies have suggested that high-risk human papillomavirus(HPV) 16/18 infection is a modifiable risk factor in development and metastasis of non-small cell lung cancer, surpassing a strong association between HIV infection and HPV-related cervical cancer, head and neck squamous cell carcinoma and oropharyngeal cancer. For example, a significant effect of HPV infection was found in lung cancer in never-smokers and women based on case-control studies[3-5]. A high frequency of oncogenic HPV types 16/18 in lung tumor tissues of nonsmoking females[3-5], the HPV16/18 E6 protein was detected in about half of HPV16/18-positive lung tumors compared with the non-controls[6-7]. In addition, a higher-frequency loss of heterozygosity (LOH) of the fragile histidine triad gene in HPV 16-infected lung tumors of females[6-7]. FHIT loss and p53 mutation may coordinate together in the development of HPV-associated lung cancer, and accelerate the occurrence and development of lung cancer[8].
In the present study, we investigated the effects of the HPV16 oncoproteins E6 and E7 on radiosensitivity of lung cancer cells and the underlying signaling pathway. We provided direct evidence that the HPV16 oncoprotein E6 or HPV16 E7 promoted the generation and secretion of vascular endothelial growth factor(VEGF) in lung cancer cells via extracellular signal-regulated kinases 1/2(ERK1/2) and AKT signaling. In addition, the HPV16 oncoproteins E6 and E7 significantly decreased the radiosensitivity of human lung cancer H2179 cells and mouse lung cancer Lewis cells. These results will help us better understand the role of high-risk HPVs in radiosensitivity and the molecular mechanisms of radioresistance in lung cancer.
-
Human lung cancer H2179 cells and mouse lung cancer Lewis cells were obtained from the Georgetown University(Washington DC, USA), and were maintained in Dulbecco′s modified Eagle′s medium(DMEM) (Gibco-BRL, USA) supplemented with 5% fetal bovine serum(FBS) and were cultured in an incubator at 37℃ with 5% CO2.
-
The p3XFLAG-HPV16 E6 and the pcDNA3FLAG-HPV16 E7 expression plasmid has been described in our previous studies[9]. Subconfluent proliferating cells in six-well dishes were incubated overnight with 1 μg of pcDNA3 FLAG-E7 or p3XFLAG-E6 expression in serum-free DMEM containing Lipofectamine 2000.
-
The transfected and untransfected cells were irradiated with various doses of 0, 2, 4, 6, 8 and 10 Gy rays using a Cs137 irradiator at a dose rate of 1 Gy/min at room temperature.
-
Cells irradiated were plated in 60-mm culture dishes at a cell density of 2×103. After 3 weeks post-irradiation, cells were fixed and stained with Giemsa solution. Colonies containing > 50 cells were counted. Colony forming efficiency=number of colonies formed/number of cells plated.
-
Western blot analysis was carried out as described before[9]. Briefly, after cells were harvested and lysed, equal amounts of protein were separated using 10% SDS-PAGE gel electrophoresis, transferred onto nitrocellulose membranes and blocked with 5% skimmed milk. The membranes were blotted with primary antibodies against the FLAG epitope(M2, mouse monoclonalantibody, Sigma, 1:500 dilution), BRCA1(C-20, rabbit polyclonal, Santa Cruz Biotechnology, 1:200), and α-actin(I-19, goat polyclonal, Santa Cruz Biotechnology, USA, 1:2000), and then incubated with HRP-conjugated anti-goat or anti-rabbit IgG antibodies(SantaCruz Biotechnology, Santa Cruz, CA, USA, 1:2000). Protein bands were visualized using the enhanced chemiluminescence detection system(Amersham Life Sciences, USA). Equality of protein loading was confirmed by fast green staining of the membrane and by immunoblotting for α-actin. Colored markers(BioRad Corp., USA) were used as molecular size standards
-
Transfected and un-transfected cells plated on 6-well plates were exposed to a 3 Gy dose of γ-ray and were then incubated at 37℃ for 0.5, 1, 2, 4 and 24 h post-irradiation. Cells were harvested and re-suspended in PBS containing chloromethyl-2', 7'-dichlorofluorescein diacetate(50 μmol/L DCFH-DA) for 15 min at 37℃. The cells were washed and incubated with 1 mg/L ROS for another 15 min. The intensity of fluorescence was assayed using the flow cytometer at 488 nm.
-
The un-transfected and transfected H2179 cells seeded on 96-well plates were cultured in serum-free DMEM for 24 h, harvested the supernatants, and VEGF levels were detected with a VEGF ELISA kit(R&D Systems, Minneapolis, MN) according to the manufacturer′s instructions. There were ten samples in each group for the ELISA.
-
Where appropriate, statistical comparisons were made using the two-tailed Student′s t test with the statistical software SPSS 13.0(SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant. The data are shown as the mean ± standard error of the mean(SEM).
-
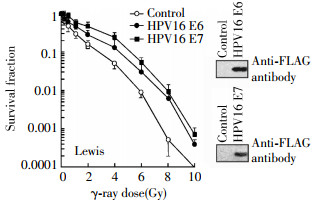
Firstly, human lung cancer H2179 cells were exposed to γ-rays at different doses(0, 2, 4, 6, 8 and 10 Gy), respectively, cells survival was analyzed by colony formation assay. As shown in Fig 1, the cell survival curve of H2179 cells transfected with a HPV16 E6 or HPV16 E7 expression vector shifted to the right, indicating the cells expressing HPV16 became more radioresistance. For example, IC90, a dose which inhibits 90% of cell survival, was 9.0 Gy(t=1.3145, P < 0.05) and 8.6 Gy(t=1.2106, P < 0.05) for HPV16 E6 and HPV16 E7 transfected cells respectively, compared to un-transfected control cells(6.2 Gy). Next, we also investigated the effects of HPV16 E6 and HPV16 E7 on the radiosensitivity in mouse lung cancer Lewis cells. As illustrated in Fig. 2, the cell survival curve of Lewis cells transfected with HPV16 E6 or HPV16 E7 expression vector also shifted to the right. The IC90 was 2.9 Gy(t=2.1154, P < 0.05) and 4.2 Gy(t=3.3970, P < 0.05) for HPV16 E6 and HPV16 E7 transfected cells respectively, compared to un-transfected control cells(1.6 Gy). These results suggest that the HPV16 E6 and HPV16 E7 oncoproteins negatively mediate the radiosensitivity of human and mouse lung cancer cells.
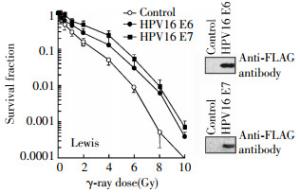
Figure 1. Negative effects of HPV16 oncoproteins E6 and E7 on radiosensitivity of human lung cancer H2179 cells. The H2179 cells were transfected with the p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid as described in the "materials and methods", irradiated at indicated doses 24 h following transfection, and subjected to clonogenic survival assay. The HPV16 oncoproteins E6 or E7 are shown in the inset Western blot by using an antiFLAG antibody.
Figure 2. Negative effects of HPV16 oncoproteins E6 and E7 on radiosensitivity of mouse lung cancer Lewis cells. The Lewis cells were transfected with a p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid as described in the "materials and methods", irradiated at indicated doses 24 h following transfection, and subjected to clonogenic survival assay. The HPV16 oncoproteins E6 or E7 are shown in the inset Western blot by using an antiFLAG antibody.
To demonstrate whether transfected cells expressed the HPV16 E6 or HPV16 E7 oncoprotein after transfection, we harvested un-transfected and transfected H2179 and Lewis cells and performed Western blot assay by using an antibody against FLAG epitope[9]. The Western blot results revealed that both H2179 and Lewis cell lines highly expressed the HPV16 E6 or HPV16 E7 oncoprotein following transfection(Figs. 1 and 2).
-
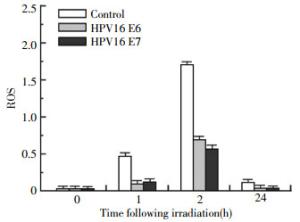
Intracellular ROS accumulation is an important marker for radiation-induced injury, thus we also studied the effects of HPV16 E6 and HPV16 E7 oncoprotein on the induction of ROS. As shown in Fig. 3, ROS levels in the cells transfected with the HPV16 E6 or HPV16 E7 expression vector became lower than the levels in the un-transfected control cells after irradiation in a dose-dependent manner. These results indicate that the radiosensitivity mediated by HPV16 E6 and HPV16 E7 is related to an inhibition of ROS.
-
VEGF is a significant mediator in radiosensitivity of lung cancer cells[10-12]. To verify whether VEGF secretion by the lung cancer cells was affected by HPV16 E6/E7, we collected supernatants of cells for the ELISA. As shown in Fig. 4, an increase of 2.3-fold(n=10, t=2.7193, P < 0.05) and 2.6-fold(n=10, t=3.1423, P < 0.05) of secreted VEGF was respectively observed in the H2179 cells expressing HPV16 E6 and HPV16 E7 compared with the un-transfected control cells at 24 h. These findings indicate that both HPV16 E6 and HPV16 E7 enhance VEGF expression.
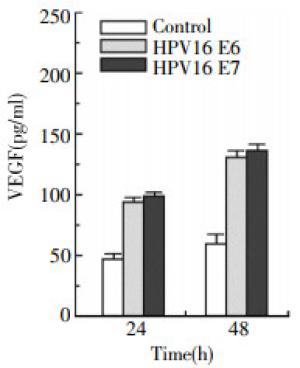
Figure 4. Effect of HPV16 oncoproteins E6 and E7 on VEGF expression. H2179 cells were transfected with p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid. After 24 or 48 h following transfection, the VEGF protein in the supernatants was detected by ELASA as described in the "materials and methods".
-
The ERK1/2 and PI3K/AKT pathways are activated in cells irradiated[13-14]. To evaluate the involvement of ERK1/2 and AKT signaling in HPV16 E6-increased VEGF expression, we compared the phosphorylation levels of ERK1/2 and AKT protein in un-transfected and transfected cells 15 min after irradiation with 3 Gy. As shown in Fig. 5 HPV16 E6 enhanced the phosphorylation of ERK1/2 caused by radiation. An enhanced AKT phosphorylation was also observed in H2179 cells expressing the HPV16 E6 oncoprotein. Similar results was obtained in the cells expressing the HPV16 E7 oncoprotein(data not shown). These results indicate that the alteration of ERK1/2 and AKT activation may be an important contributor for HPV16 E6/E7 mediated radiosensitivity.
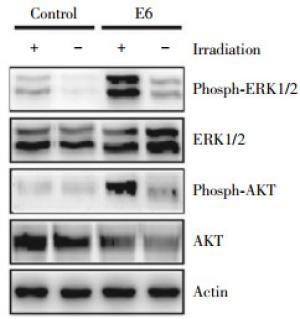
Figure 5. Activation of ERK1/2 and AKT phosphorylation by HPV16 E6/E7. Western blot was performed to assess the phosphorylation of extracellular signal-regulated kinases 1/2(ERK1/2) and AKT signaling in H2179 cells after transfection with with p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid and subjected to Western blot assay 15 min following irradiation at 3 Gy. A set of representative data was shown.
-
The HPV16/18 oncoproteins E6 and E7 are crucial for high-risk HPV-induced malignant cell transformation, such as cervical cancer. Furthermore, these oncoproteins have been proven to affect development, metastasis, chemosensitivity and radiosensitivity of human cervical cancer through different pathways[15-17]. In this study, we showed that HPV16 oncoproteins E6 and E7 significantly decreased ROS induction and increases the secretion of VEGF in H2179 cells in a time-dependent manner. This effect is mediated by the activation of the ERK1/2 and AKT signaling pathways. Furthermore, both of HPV16 E6 and E7 caused radioresistance of lung cancer cells via the ERK1/2 and PI3K/AKT pathways. These data suggested that HPV16 E6 and E7 have the potential to induce VEGF expression and decrease susceptibility of lung cancer to radiotherapy.
It has been known that induction of intracellular ROS plays an important role for apoptosis caused by radiation[18-19]. We found that HPV16 E6 and E7 along with radiation caused an elevation in the levels of intracellular ROS, which might indicate that the HPV16 E6 and E7 promotes the formation of ROS in lung cancer cells, thereby enhancing cell radiosensitivity.
VEGF is the most potent proangiogenic factor, and also contributes as important mediator to radiosensitivity of lung cancer cells[20]. As a pool of VEGF, lung cells produce and secrete VEGF in response to many stimuli, including radiation[21]. We found that the HPV16 oncoproteins E6 and E7 significantly upregulated VEGF expression in H2179 cells, and they induced VEGF expression possibly via ERK1/2 and AKT signaling, the VEGF increase last 48 h although an obvious elevation at 24 h after irradiation, this late response of VEGF expression to radiation may be that protein synthesis takes some time due to the complicated and time-consuming processes, which include DNA transcription, mRNA translation, and post-translational modification protein trafficking and secretion. The PI3K/AKT as an important signaling molecule is frequently activated in radioresistance and associated with cancer cell survival, including lung cancer[22-23]. Consistent with these previous studies, we found that the PI3K/AKT pathway also mediated the survival of lung cancer cells as well as VEGF generation after exposure to radiation, which may be required for radioresistance mediated by the HPV16 oncoproteins E6 and E7. These data suggest that the HPV16 oncoproteins E6 and E7 may exert various roles dominantly mediated by different signaling pathways, at least including activation of ERK1/2 and PI3K/AKT pathway as well as VEGF signaling.
In summary, our present study, for the first time, show that the radiosensitivity of lung cancer cells may be modulated by the HPV16 oncoproteins E6 and E7. The mechanism involved the radioresistance might be related to ROS formation, promotion of ERK1/2 and PI3K/AKT signaling pathways and VEGF expression. These findings provide new insight into an important role of the HPV16 oncoproteins E6 and E7 as potential radiosensitivity mediators in lung cancer, excluding their roles as modifiable risk factors in lung carcinogenesis.
HPV16 E6/E7 Negatively Affect Radiosensitivity of Lung Cancer Cells
HPV16 E6/E7 Negatively Affect Radiosensitivity of Lung Cancer Cells
-
-
关键词:
- Papillomavirus, human /
- Lung neoplasms /
- Radiosensitivity /
- ERK1/2 and AKT /
- ROS
Abstract:Objective Lung cancer cells associated with radioresistance are likely to give rise to local recurrence and distant metastatic relapse, but little is known about its underlying mechanisms. In the present paper, the effects of the HPV16 E6 and HPV16 E7 oncoprotein on the radiosensitivity of lung cancer cell lines were investigated. Methods The HPV16 E6 or HPV16 E7 oncoprotein was expressed by a transient transfection with pcDNA3-HPV16 E6 or pcDNA3-HPV16 E7 expression vector. Human lung cancer H2179 cells and mouse lung cancer Lewis cells were exposed to a γ-ray radiation source, cellular survival was evaluated by using a colony formation assay. The expression of HPV16 oncoproteins E6/E7, extracellular signal-regulated kinases 1/2(ERK1/2) and AKT signaling was determined by Western blot assay. VEGF secretion was determined by ELISA. Results Both HPV16 oncoproteins E6 and E7 significantly decreased radiosensitivity of H2179 cells, associated with a promotion of the ERK1/2 and AKT phosphorylation. A decrease of reactive oxygen species(ROS) and an increase of VEGF levels were observed in the cells expressing the HPV16 oncoproteins E6 and E7. Furthermore, a similar reduction of radiosensitivity mediated by the HPV16 oncoproteins E6 and E7 was also observed in a mouse lung cancer Lewis cells. Conclusion The findings indicate that the HPV16 oncoproteins E6 and E7 negatively affects susceptibility of lung cancer cells to radiotherapy via regulation of the ERK1/2 and Akt signaling pathway and VEGF expression. -
Key words:
- Papillomavirus, human /
- Lung neoplasms /
- Radiosensitivity /
- ERK1/2 and AKT /
- ROS
-
Figure 1. Negative effects of HPV16 oncoproteins E6 and E7 on radiosensitivity of human lung cancer H2179 cells. The H2179 cells were transfected with the p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid as described in the "materials and methods", irradiated at indicated doses 24 h following transfection, and subjected to clonogenic survival assay. The HPV16 oncoproteins E6 or E7 are shown in the inset Western blot by using an antiFLAG antibody.
Figure 2. Negative effects of HPV16 oncoproteins E6 and E7 on radiosensitivity of mouse lung cancer Lewis cells. The Lewis cells were transfected with a p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid as described in the "materials and methods", irradiated at indicated doses 24 h following transfection, and subjected to clonogenic survival assay. The HPV16 oncoproteins E6 or E7 are shown in the inset Western blot by using an antiFLAG antibody.
Figure 4. Effect of HPV16 oncoproteins E6 and E7 on VEGF expression. H2179 cells were transfected with p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid. After 24 or 48 h following transfection, the VEGF protein in the supernatants was detected by ELASA as described in the "materials and methods".
Figure 5. Activation of ERK1/2 and AKT phosphorylation by HPV16 E6/E7. Western blot was performed to assess the phosphorylation of extracellular signal-regulated kinases 1/2(ERK1/2) and AKT signaling in H2179 cells after transfection with with p3XFLAG-HPV16 E6 or pcDNA3FLAG-HPV16 E7 expression plasmid and subjected to Western blot assay 15 min following irradiation at 3 Gy. A set of representative data was shown.
-
[1] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2):115-132. DOI:10.3322/caac.21338. [2] Tsao AS, Scagliotti GV, Bunn PA Jr, et al. Scientific Advances in Lung Cancer 2015[J/OL]. J Thorac Oncol, 2016[2016-03-01]. http://www.ncbi.nlm.nih.gov/pubmed/27013409. DOI: 10.1016/j.jtho.2016.03.012. [3] Cheng YW, Lin FC, Chen CY, et al. Environmental exposure and HPV infection may act synergistically to induce lung tumorigenesis in nonsmokers[J/OL]. Oncotarget, 2016[2016-03-01]. http://www.ncbi.nlm.nih.gov/pubmed/?term=26918347. DOI: 10.18632/oncotarget.7628. [4] Bae JM, Kim EH. Human papillomavirus infection and risk of lung cancer in never-smokers and women: an 'adaptive' meta-analysis[J/OL]. Epidemiol Health, 2015, 37: e2015052[2016-03-01]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4722221. DOI: 10.4178/epih/e2015052. [5] Bae JM. Modifiable risk factors of lung cancer in "never-smoker" women[J/OL]. Epidemiol Health, 2015, 37: e2015047[2016-03-01]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4722226. DOI: 10.4178/epih/e2015047. [6] Zhai K, Ding J, Shi HZ. HPV and lung cancer risk: a meta-analysis[J/OL]. J Clin Virol, 2015, 63: 84-90. DOI: 10.1016/j.jcv.2014.09.014. [7] Ragin C, Obikoya-Malomo M, Kim S, et al. HPV-associated lung cancers:an international pooled analysis[J]. Carcinogenesis, 2014, 35(6):1267-1275. DOI:10.1093/carcin/bgu038. [8] Yu Y, Liu X, Yang Y, et al. Effect of FHIT loss and p53 mutation on HPV-infected lung carcinoma development[J]. Oncol Lett, 2015, 10(1):392-398. DOI:10.3892/ol.2015.3213. [9] Zhang Y, Fan S, Meng Q, et al. BRCA1 interaction with human papillomavirus oncoproteins[J]. J Biol Chem, 2005, 280(39):33165-33177. DOI:10.1074/jbc.M505124200. [10] Barker HE, Paget JT, Khan AA, et al. The tumour microenvironment after radiotherapy:mechanisms of resistance and recurrence[J]. Nat Rev Cancer, 2015, 15(7):409-425. DOI:10.1038/nrc3958. [11] Drigotas M, Affolter A, Mann WJ, et al. Reactive oxygen species activation of MAPK pathway results in VEGF upregulation as an undesired irradiation response[J]. J Oral Pathol Med, 2013, 42(8):612-619. DOI:10.1111/jop.12056. [12] Gao X, Zhao Y, Stemmer-Rachamimov AO, et al. Anti-VEGF treatment improves neurological function and augments radiation response in NF2 schwannoma model[J]. Proc Natl Acad Sci USA, 2015, 112(47):14676-14681. DOI:10.1073/pnas.1512570112. [13] Ciccarelli C, Vulcano F, Milazzo L, et al. Key role of MEK/ERK pathway in sustaining tumorigenicity and in vitro radioresistance of embryonal rhabdomyosarcoma stem-like cell population[J/OL]. Mol Cancer, 2016, 15(1): 16[2016-03-01]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4761200. DOI: 10.1186/s12943-016-0501-y. [14] Provencio M, Sánchez A. Therapeutic integration of new molecule-targeted therapies with radiotherapy in lung cancer[J]. Transl Lung Cancer Res, 2014, 3(2):89-94. DOI:10.3978/j.issn.2218-6751. 2014.03.06. [15] Marullo R, Werner E, Zhang H, et al. HPV16 E6 and E7 proteins induce a chronic oxidative stress response via NOX2 that causes genomic instability and increased susceptibility to DNA damage in head and neck cancer cells[J]. Carcinogenesis, 2015, 36(11):1397-1406. DOI:10.1093/carcin/bgv126. [16] Tsang NM, Nagasawa H, Li C, et al. Abrogation of p53 function by transfection of HPV16 E6 gene enhances the resistance of human diploid fibroblasts to ionizing radiation[J]. Oncogene, 1995, 10(12):2403-2408. [17] Vozenin MC, Lord HK, Hartl D, et al. Unravelling the biology of human papillomavirus(HPV) related tumours to enhance their radiosensitivity[J]. Cancer Treat Rev, 2010, 36(8):629-636. DOI:10.1016/j.ctrv.2010.03.010. [18] Malik A, Sultana M, Qazi A, et al. Role of natural radiosensitizers and cancer Cell Radioresistance: An Update[J/OL]. Anal Cell Pathol (Amst), 2016, 2016: 6146595[2016-03-01]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4779816. DOI: 10.1155/2016/6146595. [19] Kim W, Youn H, Kang C, et al. Inflammation-induced radioresistance is mediated by ROS-dependent inactivation of protein phosphatase 1 in non-small cell lung cancer cells[J]. Apoptosis, 2015, 20(9):1242-1252. DOI:10.1007/s10495-015-1141-1. [20] Chen YH, Pan SL, Wang JC, et al. Radiation-induced VEGF-C expression and endothelial cell proliferation in lung cancer[J]. Strahlenther Onkol, 2014, 190(12):1154-1162. DOI:10.1007/s00066-014-0708-z. [21] Fu ZZ, Sun XD, Li P, et al. Relationship between serum VEGF level and radiosensitivity of patients with nonsmall cell lung cancer among asians:a meta-analysis[J]. DNA Cell Biol, 2014, 33(7):426-437. DOI:10.1089/dna.2013.2249. [22] Tang Y, Liu B, Li J, et al. Genetic variants in PI3K/AKT pathway are associated with severe radiation pneumonitis in lung cancer patients treated with radiation therapy[J]. Cancer Med, 2016, 5(1):24-32. DOI:10.1002/cam4.564. [23] Lei Y, Li HX, Jin WS, et al. The radiosensitizing effect of Paeonol on lung adenocarcinoma by augmentation of radiation-induced apoptosis and inhibition of the PI3K/Akt pathway[J]. Int J Radiat Biol, 2013, 89(12):1079-1086. DOI:10.3109/09553002.2013.825058. -





 下载:
下载: