-
Radiotherapy has a crucial role in the treatment of non-small cell lung cancer[1]. However, esophageal toxicity and pulmonary toxicity are common toxic side effects of radiotherapy and usually interrupt its planned course[2]. Acute esophageal toxicity during the course of treatment can disrupt normal activities, such as swallowing, drinking, and eating, of patients. Pulmonary toxicity causes coughing, aggravates sputum production, and induces posterior sternal pain. Thus, esophageal and pulmonary toxicities cause the life quality of patients to deteriorate.
Numerous drugs with the potential to protect normal tissues from intensive radiotherapy and/or chemotherapy while exerting the optimal therapeutic effect have been investigated over the past several decades. Amifostine is an organic thiophosphate pro-drug that is dephosphorylated in vivo into its active moiety, WR-1065(5); it has been developed to selectively protect normal tissues against the toxic effects of radiotherapy and/or chemotherapy by scavenging free radicals[3]. Some randomized controlled trials(RCTs) have demonstrated that amifostine could reduce the risk of esophageal toxicity and pulmonary toxicity in patients with lung cancer and receiving radiation or concomitant chemoradiotherapy[4-5]. However, some RCTs have shown that amifostine cannot reduce radiation toxicities[6-7]. Some investigators have even suggested that amifostine can reduce the therapeutic effects of radiation or chemotherapy by exerting tumor-protective effects[8].
Thus far, however, the radiotherapy and/or chemotherapy protection efficacy of amifostine lacks adequate statistical support. We performed this systematic review and meta-analysis to confirm whether amifostine can reduce the risk of radiotherapy and/or chemotherapy toxicities and to evaluate its therapeutic efficacy in lung cancer.
-
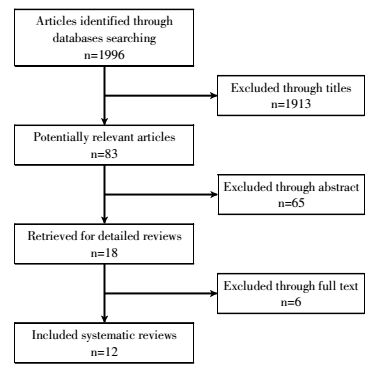
The procedure for study selection is shown in Fig. 1. The electronic databases PubMed, Cochrane Central Register of Controlled Trials, EMBASE, and China National Knowledge Infrastructure were comprehensively searched for articles that were published over the period of January 1, 2000 to December 31, 2016. The following search terms were used: "lung cancer", "WR2721" and "amifostine". Search languages were limited to English and Chinese. All references of relevant articles were scanned for additional articles.
-
Details regarding the patients′ eligibility criteria, treatment methods, and outcomes of the relevant trials were extracted by two reviewers(Huanan Wang and Feng Wang) and then checked by the third reviewer (Yonghan Wang) in accordance with the Cochrane Handbook for systematic reviews(version 5.1.0)[9]. The patient population was limited to patients with lung cancer. The intervention was radiotherapy or chemoradiotherapy plus amifostine, and the control intervention was radiotherapy or chemoradiotherapy. Outcomes were restricted to esophageal toxicity, pulmonary toxicity, response rate, overall survival rate, hematological toxicity, and amifostine-related side effects. RCTs that included patients with lung cancer and other kinds of tumors were also included in the meta-analysis. However, data were extracted only for patients with lung cancer.
-
Data were independently extracted by two reviewers (Huanan Wang and Yonghan Wang) from all included RCTs. Another investigator (Feng Wang) was consulted to resolve any disagreements. The general characteristics (name of the first author, year of publication, number of patients, stages, chemoradiation regimens, and amifostine dosage), outcomes(esophageal toxicity, pulmonary toxicity, response rate, overall survival rate, hematological toxicity, and amifostine-related side effects) were extracted. The methodological qualities of the trials were assessed by the same investigators (Huanan Wang and Yonghan Wang) in accordance with the Cochrane Reviews Handbook 5.1.0. Allocation concealment, binding of participants and personnel, random sequence generation, binding of outcome assessment, incomplete data outcome, and selective reporting received special attention during the trial inclusion procedure given that these factors represent the quality of the RCT[9].
-
All analyses were performed strictly with Review Manager (RevMan 5.3, provided by The Cochrane Collaboration). Dichotomous data were calculated as the risk ratio (RR) with 95% confidence interval (CI). The null hypothesis was considered as no association(RR=1) between amifostine use and the incidence of chemoradiation toxicity or the tumor response rate. RR < 1 indicated that amifostine positively affected the outcome. The statistical heterogeneity of the results across trials was assessed through χ2 test[10], and inconsistency was calculated through I2 test[11]. If heterogeneity was present (χ2, P < 0.05, or I2>50%), data were pooled through the random-effect method(Dersimonian-Laird method). Subgroup analysis was conducted for further evaluation. The fixed-effect method was used if significant heterogeneity was absent. Egger′s tests were used for each effect size to evaluate possible publication bias as described by Egger[12].
-
Trials No. of patients
Ami/ControlStage
includedDaily ami
(dose)Administration Concomitant
chemotherapyRadiotherapy Zhao(2014)[5] 69/68 Ⅲ, Ⅳ 200 mg/m2 Ⅳ, 30 min before RT, q.d 54-66 Gy, 2 Gy/fraction, 5 fractions/week Lin(2013)[13] 21/23 Ⅱ, Ⅲ 300 mg/m2 Ⅳ, 15-30 min before RT, q.d 54 Gy, 1.8 Gy/fraction, 5 fractions weekly Liu(2015)[4] 25/25 Ⅲ 300 mg/m2 Ⅳ, 30 min before RT, q.d DDP (50 mg/m2) + E (50 mg/m2)daily for the first 4 weeks of RT. DDP (50 mg/m2) + E(50 mg/m2)/DDP(50 mg/m2) + V(50 mg/m2)/C(50 mg/m2) + P(50 mg/m2) daily for the second 4 weeks of RT 66 Gy, 16.5 Gy/week Li(2010)[14] 55/53 Ⅱ, Ⅲ, Ⅳ 200 mg/m2 Ⅳ, 30 min before RT, q.d 54-66 Gy, 2 Gy/fraction, 5 fractions/week Weng(2007)[15] 30/30 Ⅲ 300 mg/m2 Ⅳ, 30 min before RT, q.d P (135 mg/m2) days: 1 + DDP(50 mg/m2) days:1-3 50-60 Gy, 2 Gy/fraction, 5 fractions/week Movsas(2005)[6] 114/115 Ⅲ 500 mg, 4 times/ week Ⅳ, 15-30 min before RT q.d, on RT-onlydays; 180minbefore RT q.d, on CT + RT days P(225 mg/m2) + C(AUC6) days: 1, 22; P(50mg/m2) + C (AUC2) days:43, 50, 57, 64, 71, 78 69.6 Gy, 1.2 Gy bid, 5 days/week Komaki(2004)[16] 31/31 Ⅱ, Ⅲ 500 mg, 2 times/week Ⅳ, 20-30 min before CT q.d, days: 1, 8, 29, 36; 60-90 minbefore first fraction RT; 30-60 min before RT q.d, days: 2, 9, 30, 37 E(50mg/m2)days: 1-10, 29-38DDP(50mg/m2)days: 1, 8, 29, 36 69.6 Gy, 1.2 Gy bid, 5 days/week Leong (2003)[17] 30/30 Ⅲ 740 mg/m2 Ⅳ, 30 min before CT q.d P(175 mg/m2) + C (AUC6) days: 1, 22; P(60 mg/m2) days: 43, 50, 57, 64, 71, 78 60-66 Gy, 2 Gy fraction, 5 fractions/week Antonadou(2003)[18] 36/32 Ⅲ 300 mg/m2 Ⅳ, 15 min before RT and before CT on CT days q.d P(60 mg/m2) / C(AUC2) weekly before RT 55-60 Gy, 2 Gy fraction, 5 fractions/week Senzer(2002)[7] 24/25 Ⅲ 500 mg/ 200 mg q.d Ⅳ, 500 mg, 15-30 min weekly before CT; 200 mg/m2, 15 -30 min before RT (including the day of CT) q.d P(50 mg/m2) + C(AUC2) weekly +G(1000 mg/m2) days: 22, 29, 36+DDP(80 mg/m2) days: 29 beforeRT 64.8 Gy, 36 fractions over 7.5 weeks Antonadou(2001)[19] 44/53 Ⅲ 340 mg/m2 Ⅳ, 15 min before RT q.d 55-60 Gy, 2 Gy fraction, 5 fractions/week Koukourakis(2001)[20] 19/17 Ⅲ 500 mg IH, 20 min before RT q.d 64 Gy, 2 Gy/fraction, 5 fractions/week Notes: Ami=amifostine; IV=intravenous injection; IH=subcutaneously injected; RT=radiotherapy; C=carboplatin; G=gemcitabine; V=vinorelbine; DDP=cisplatin; P=paclitaxel; E=etoposide; AUC=area under the curve; qd=daily; bid=twice daily. Table 1. General characteristics of included radomized controlled trials
For the entire patient population, 1996 articles were retrieved through the initial search. After reviewing titles and abstracts, 1778 articles were removed. The full texts of the remaining 12 articles were reviewed for inclusion in the meta-analysis. All twelve trials were RCTs and published in English or Chinese[4-7, 13-20] in the period of 2000 to 2016. The included RCTs involved 1000 patients(604 and 563 in each treatment arm).
Methodological quality was evaluated with a seven-question instrument described in the Cochrane Reviews Handbook 5.1.0. Generally, the 12includedtrials were considered to be at moderate risk of bias. Although randomization was performed in all 12 trials, only two articles mentioned allocation concealment[16, 18]. In addition, all 12 trials performed an adequate sequence generation[4-7, 13-20]. Only one trial described blinding patients and physicians or evaluators. The outcome of methodological quality for each trial is presented in Fig. 2.
-
Of the 12 trials, nine[4, 6-7, 13, 15-19] trials evaluated acute esophageal toxicity with evident heterogeneity between studies(I2=84%) (Fig. 3). The meta-analysis was performed using the random-effect model(Dersimonian-Laird method). Pooled analysis showed that the use of amifostine reduced acute esophageal toxicity by 44% (RR, 0.56; 95%CI, 0.39-0.81; P=0.002). Egger′s test revealed that publication bias was absent(P=0.206).Subgroup analysis indicated that the use of amifostine significantly reduced acute esophageal toxicity in patients receiving concurrent chemoradiation(RR, 0.67;95%CI, 0.49-0.93; P=0.020) and radiation only(RR, 0.20; 95%CI, 0.05-0.80; P=0.020)(Table 2).

Figure 3. Forest plot of acute esophageal toxicity(all grades) in patients with lung cancer who received radiotherapy or concomitant chemoradiation
Subgroup Acute esophageal P Acute pulmonary P Late pulmonary P RR 95%CI RR 95%CI RR 95%CI Chemoradiation 0.67 0.49-0.93 0.020 0.44 0.21-0.95 0.040 0.84 0.48-1.46 0.540 Radiation only 0.20 0.05-0.80 0.020 0.38 0.25-0.58 0.001 Table 2. Subgroup analysis of radiation-induced side effects in accordance with treatment strategy
-
Nine articles[4-7, 13-14, 16, 18-19] reported the number of patients who developed acute pulmonary toxicity in both treatment arms. Heterogeneity was observed among trials. Pooled analysis with the random-effect model demonstrated that amifostine reduced all grades ofacute pulmonary toxicity in patients with lung cancer (RR, 0.42; 95%CI, 0.25-0.70; P=0.001)(Fig. 4). Egger′s test revealed the absence of publication bias (P=0.244). Subgroup analysis revealed that the use of amifostine significantly reduced acute pulmonary toxicity in patients treated with concurrent chemoradiation(RR, 0.44; 95%CI, 0.21-0.95; P=0.040) and radiation only (RR, 0.38; 95% CI, 0.25-0.58; P < 0.001)(Table 2).
-
Late pulmonary toxicity was reported in three trials[6, 18-19] with heterogeneity among studies (I2=59%). Meta-analysis showed that the risk (RR, 0.74; 95%CI, 0.45-1.19; P=0.210) of late pulmonary toxicity was not significantly lower in the amifostine group than that in the control treatment (Fig. 5). Publication bias was not observed by Egger′s test (P=0.052). Subgroup analysis also showed that the use of amifostine did not reduce the risk of pulmonary toxicity in lung cancer patients treated with concurrent chemoradiation (RR, 0.84; 95%CI, 0.48-1.46; P=0.540) (Table 2).
-
Data on hematological toxicity, including neutropenia and thrombocytopenia, were extracted from five articles[6, 15-18]. Studies involving neutropenia exhibited heterogeneity (I2=74%). However, studies involving thrombocytopenia were not heterogeneous(I2=0%). Meta-analysis showed that the incidences of neutropenia (RR, 1.02; 95%CI, 0.61-1.71; P=0.940) in the amifostine and control groups were not significantly different. Egger′s test revealed no publication bias in this subset analysis(P=0.182). The use of amifostine significantly reduced the incidence of thrombocytopenia(RR, 0.45; 95%CI, 0.21-0.94; P=0.030)(Fig. 6).
-
Nine articles provided response rates[4-6, 13, 15, 17-20]. No statistical heterogeneity among studies was found in both complete(I2=0%) and partial(I2=0%) response analysis. The pooled RR estimate for partial response was 0.98(95%CI, 0.83-1.15; P=0.800) (Fig. 6), which was not statistically significant. Publication bias was not observed through Egger′s test(P=0.138). The pooled RR estimate for the complete response was 1.50(95%CI, 1.03-2.18; P=0.030) and was statistically significant (Fig. 7). However, publication bias was observed through Egger′s test (P=0.000).
-
Three articles reported overall survival rates[6, 16-17]. No statistical heterogeneity among studies was found in both one-year overall survival(I2=0%) and two-year overall survival(I2=0%) analysis. The pooled RR estimate for the one-year overall survival was 0.94(95%CI, 0.81-1.09; P=0.400). Publication bias was not observed through Egger′s test(P=0.555). The pooled RR estimate for two-year overall survival was 1.06(95%CI, 0.81-1.39; P=0.680) (Fig. 8). Publication bias was not observed through Egger′s test(P=0.732). Neither one-year overall survival nor two-year overall survival reached statistical significance (Fig. 8).
-
Six studies[14-19] described amifostine toxicity. The most common amifostine-related side effects included nausea, vomiting, and transient hypotension with average incidence rates of 11%, 14%, and 24%, respectively. However, amifostine toxicity can be controlled through clinical treatment or resting.
-
In 2016, lung cancer became the leading cause of cancer-related deaths worldwide and accounted for more than 21% of all cancer-related deaths. Most lung cancer cases are diagnosed at advanced stages[21] and thus could not be treated through surgery. Chemotherapy with radiotherapy may be the most effective treatment strategy against lung cancer[22]. However, patients inevitably experience serious radiation-related toxicities, such as esophageal toxicity and pulmonary toxicity, as they receive increasing radiotherapy doses. In the 1950s, amifostine, a thiol-containing radioprotector, was initially developed as part of the nuclear warfare program. The cytoprotective mechanism of amifostine is complicated and involves free-radical scavenging, DNA protection and repair acceleration, and cellular hypoxia induction[23]. The US Food and Drug Administration has approved the use of amifostine as a cytoprotector in cisplatin chemotherapy and radiation-induced xerostomia[23]. However, whether amifostine can attenuate the severity of radiation-related toxicity without exerting tumor-protective effects remains controversial. Thus, we performed this systematic review and meta-analysis to compile inconsistent evidence for the assessment of the true clinical efficacy of this drug.
In this meta-analysis, we found that use of amifostine significantly reduced radiation-induced acute esophageal toxicity(P=0.002) and acute pulmonary toxicity(P=0.001). Subgroup analysis showed that the use of amifostine significantly reduced acute esophageal toxicity(P=0.020) and acute pulmonary toxicity (P < 0.001) in patients receiving concurrent chemoradiation(P=0.020) and radiation only(P=0.020)(Table 2). However, the use of amifostine did not reduce late ulmonary toxicity(P=0.210). Subgroup analysis demonstrated that patients receiving concomitant chemoradiation do not derive benefit from amifostine in terms of reduced late pulmonary toxicity. Thus, amifostine can reduce acute esophageal toxicity and pulmonary toxicity in patients receiving concomitant chemoradiation and radiation only but cannot reduce late pulmonaryin patients receiving concomitant chemoradiation.
A major controversy for the clinical use of amifostine is its potential tumor-protective effect. Several pharmacological experiments have indicated that amifostine may exert a protective effect on tumor tissues by a lower degree than on normal tissues[8]. Some RCTs have shown that amifostine does not significantly influence treatment response[5, 17]. However, considering the realities of RCTs and clinical practice, absolutely negating the tumor-protective effects of amifostineis difficult. Therefore, we performed a meta-analysis to obtain an objective result from repeatedly inconsistent trials. We found no statistically significant difference in partial response(P=0.800) between the two treatment arms. Although we found that amifostine improved complete response(P=0.030), we observed publication bias through Egger′s test(P=0.000). Moreover, we did not find a statistically significant difference in one-year overall survival(P=0.400) and two-yeary overall survival (P=0.680). Therefore, we concluded that amifostine does not exert tumor-protective effects in radiation therapy.
Another controversial issue about amifostine is its related toxicities. The most common side effects of this drug include nausea, vomiting, or transient hypotension with incidences of 2%-70%[14-19]. Our study showed that the average incidences of nausea, vomiting, and hypotension are 11%, 14% and 24% respectively. However, amifostine toxicity can be controlled through clinical treatment or resting.
The results of this meta-analysis were based on published RCTs and not on the data of individual patients. Our results should therefore be interpreted with caution. Save for the result of complete response, no evidence of publication bias was observed through Egger′s test. Nevertheless, given the small number of trials and possible existence of unpublished studies, publication bias may be difficult to exclude completely.
Similar reviews of amifostine have been published in the past. Compared with previous studies, we included more updated RCTs in our study. We retrieved 1996 articles involving the entire patient population from the initial search. We ultimately analyzed 12 RCTs involving 1000 patients with lung cancer. The full texts ofall 12 trials were published in English or Chinese and were published in the period of 2000 to 2016. We then performed a subgroup analysis of radiation-induced side effects in accordance with treatment strategy. Therefore, our meta-analysis may be more comprehensive and credible than previous meta-analyses given that we included a higher number of patients and we conducted subgroup analysis for further evaluation.
In conclusion, this systematic review demonstrated that the concurrent administration of amifostine with radiotherapy to patients with lung cancer significantly reduces acute esophageal toxicity, pulmonary toxicity, and thrombocytopenia without exerting any tumor-protective effects. Amifostine-related toxicities can be controlled through clinical treatment or resting. We should weigh the beneficial effects of the reduction in radiation-induced toxicities against the adverse effects of amifostine-related toxicities in accordance with individual treatment strategy. Our results indicated that amifostine has a continuously expanding role inradiation therapy. Well-designed RCTs are essential for exploring the potential benefits of amifostine in the future.
Effect of Amifostine on Patients with Lung Cancer Treated with Radiotherapy or Concomitant Chemoradiotherapy:A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Effect of Amifostine on Patients with Lung Cancer Treated with Radiotherapy or Concomitant Chemoradiotherapy:A Systematic Review and Meta-Analysis of Randomized Controlled Trials
-
Abstract:
ObjectiveAmifostine is clinically used as a chemical radioprotector. Nevertheless, its efficacy as a radioprotector remains controversial. MethodsPubMed, Cochrane Central Register of Controlled Trials, EMBASE, China National Knowledge Infrastructure, and the references of the published results of trials on the efficacy of amifostine in patients with lung cancer and who received radiotherapy or concomitant chemoradiotherapy were searched. The pooled radiation protection efficacy, treatment response, and side effects of amifostine were calculated using RevMan software. ResultsTwelve randomized controlled trials involving 1000 patients with lung cancer were ultimately analyzed. Results of meta-analysis revealed that the use of amifostine reduced the risk of acute esophageal toxicity(RR, 0.56; 95% CI, 0.39-0.81; P=0.002) and pulmonary toxicity(RR, 0.42; 95% CI, 0.25-0.70; P=0.001). Subgroup analysis also demonstrated that the risk of acute esophageal toxicity and pulmonary toxicity significantly reduced in patients who received chemoradiation concurrent with amifostine or radiation only. Pooled data showed that the use of amifostine did not significantly decrease the risk of late pulmonary toxicity(RR, 0.74; 95% CI, 0.45-1.19; P=0.210). Moreover, subgroup analysis demonstrated that the risk oflate pulmonary toxicity did not significantly decrease in patients who received chemoradiotherapy concomitant with amifostine(RR, 0.84; 95% CI, 0.48-1.46; P=0.540). Amifostine did not exert tumor-protective effects in partial response(RR, 0.98; 95% CI, 0.83-1.15; P=0.800) but improved complete response(RR, 1.50; 95% CI, 1.03-2.18; P=0.030), although publication bias was observed through Egger's test(P=0.000). Moreover, amifostine had no effect on one-year overall survival (RR, 0.94; 95% CI, 0.81-1.09; P=0.400) and two-year overall survival(RR, 1.06; 95% CI, 0.81-1.39; P=0.680) rates. The incidence of neutropenia, a hematologic side effect of amifostine, was not significantly different(RR, 1.02; 95% CI, 0.61-1.71; P=0.940) between the amifostine and control group. The use of amifostine, however, significantly decreased the incidence of thrombocytopenia(RR, 0.45; 95% CI, 0.21-0.94; P=0.030). The most common amifostine-related side effects were nausea, vomiting, and hypotension with average incidence rates of 11%, 14%, and 24%, respectively. ConclusionsThis systematic review showed that the concurrent administration of amifostine with radiotherapy to patients with lung cancer significantly reduced the risks of acute esophageal toxicity and acute pulmonary toxicity and decreased the incidence of thrombocytopenia without tumor-protecting effects. In addition, the toxicities of amifostine were generally controllable through clinical treatment or resting. -
Key words:
- Amifostine /
- Lung neoplasms /
- Radiotherapy /
- Concomitant chemoradiotherapy /
- Meta-analysis
-
Table 1. General characteristics of included radomized controlled trials
Trials No. of patients
Ami/ControlStage
includedDaily ami
(dose)Administration Concomitant
chemotherapyRadiotherapy Zhao(2014)[5] 69/68 Ⅲ, Ⅳ 200 mg/m2 Ⅳ, 30 min before RT, q.d 54-66 Gy, 2 Gy/fraction, 5 fractions/week Lin(2013)[13] 21/23 Ⅱ, Ⅲ 300 mg/m2 Ⅳ, 15-30 min before RT, q.d 54 Gy, 1.8 Gy/fraction, 5 fractions weekly Liu(2015)[4] 25/25 Ⅲ 300 mg/m2 Ⅳ, 30 min before RT, q.d DDP (50 mg/m2) + E (50 mg/m2)daily for the first 4 weeks of RT. DDP (50 mg/m2) + E(50 mg/m2)/DDP(50 mg/m2) + V(50 mg/m2)/C(50 mg/m2) + P(50 mg/m2) daily for the second 4 weeks of RT 66 Gy, 16.5 Gy/week Li(2010)[14] 55/53 Ⅱ, Ⅲ, Ⅳ 200 mg/m2 Ⅳ, 30 min before RT, q.d 54-66 Gy, 2 Gy/fraction, 5 fractions/week Weng(2007)[15] 30/30 Ⅲ 300 mg/m2 Ⅳ, 30 min before RT, q.d P (135 mg/m2) days: 1 + DDP(50 mg/m2) days:1-3 50-60 Gy, 2 Gy/fraction, 5 fractions/week Movsas(2005)[6] 114/115 Ⅲ 500 mg, 4 times/ week Ⅳ, 15-30 min before RT q.d, on RT-onlydays; 180minbefore RT q.d, on CT + RT days P(225 mg/m2) + C(AUC6) days: 1, 22; P(50mg/m2) + C (AUC2) days:43, 50, 57, 64, 71, 78 69.6 Gy, 1.2 Gy bid, 5 days/week Komaki(2004)[16] 31/31 Ⅱ, Ⅲ 500 mg, 2 times/week Ⅳ, 20-30 min before CT q.d, days: 1, 8, 29, 36; 60-90 minbefore first fraction RT; 30-60 min before RT q.d, days: 2, 9, 30, 37 E(50mg/m2)days: 1-10, 29-38DDP(50mg/m2)days: 1, 8, 29, 36 69.6 Gy, 1.2 Gy bid, 5 days/week Leong (2003)[17] 30/30 Ⅲ 740 mg/m2 Ⅳ, 30 min before CT q.d P(175 mg/m2) + C (AUC6) days: 1, 22; P(60 mg/m2) days: 43, 50, 57, 64, 71, 78 60-66 Gy, 2 Gy fraction, 5 fractions/week Antonadou(2003)[18] 36/32 Ⅲ 300 mg/m2 Ⅳ, 15 min before RT and before CT on CT days q.d P(60 mg/m2) / C(AUC2) weekly before RT 55-60 Gy, 2 Gy fraction, 5 fractions/week Senzer(2002)[7] 24/25 Ⅲ 500 mg/ 200 mg q.d Ⅳ, 500 mg, 15-30 min weekly before CT; 200 mg/m2, 15 -30 min before RT (including the day of CT) q.d P(50 mg/m2) + C(AUC2) weekly +G(1000 mg/m2) days: 22, 29, 36+DDP(80 mg/m2) days: 29 beforeRT 64.8 Gy, 36 fractions over 7.5 weeks Antonadou(2001)[19] 44/53 Ⅲ 340 mg/m2 Ⅳ, 15 min before RT q.d 55-60 Gy, 2 Gy fraction, 5 fractions/week Koukourakis(2001)[20] 19/17 Ⅲ 500 mg IH, 20 min before RT q.d 64 Gy, 2 Gy/fraction, 5 fractions/week Notes: Ami=amifostine; IV=intravenous injection; IH=subcutaneously injected; RT=radiotherapy; C=carboplatin; G=gemcitabine; V=vinorelbine; DDP=cisplatin; P=paclitaxel; E=etoposide; AUC=area under the curve; qd=daily; bid=twice daily. Table 2. Subgroup analysis of radiation-induced side effects in accordance with treatment strategy
Subgroup Acute esophageal P Acute pulmonary P Late pulmonary P RR 95%CI RR 95%CI RR 95%CI Chemoradiation 0.67 0.49-0.93 0.020 0.44 0.21-0.95 0.040 0.84 0.48-1.46 0.540 Radiation only 0.20 0.05-0.80 0.020 0.38 0.25-0.58 0.001 -
[1] Senan S, Lagerwaard FJ. The role of radiotherapy in non-small-cell lung cancer[J]. Ann Oncol, 2005, 16 suppl 2:S223-228. DOI:10.1093/annonc/mdi726. [2] Ozgen A, Hayran M, Kahraman F. Mean esophageal radiation dose is predictive of the grade of acute esophagitis in lung cancer patients treated with concurrent radiotherapy and chemotherapy[J].J Radiat Res, 2012, 53(6):916-922. DOI:10.1093/jrr/rrs056. [3] Walker DM, Kajon AE, Torres SM, et al. WR1065 mitigates AZT-ddI-Induced mutagenesis and inhibits viral replication[J]. Environ Mol Mutagen, 2009, 50(6):460-472. DOI:10.1002/em.20482. [4] 刘彩彩.局部晚期非小细胞肺癌放疗中阿米福汀的作用研究[J].母婴世界, 2015, 15:16.
Liu CC. Study on the effect of amifostine in local advanced NSCLC radiotherapy[J]. Mother Child World, 2015, 15:16.[5] 赵衍.阿米福汀在非小细胞肺癌放疗中对正常组织的保护研究[J].临床肺科杂志, 2014, 19(11):2050-2052. DOI:10.3969/j.issn.1009-6663. 2014.011.036.
Zhao Y. Amifostine protective effect for normal tissue in the radiotherapy of non-small cell lung cancer[J]. J Clinic Pulmonary Med, 2014, 19(11):2050-2052. doi: 10.3969/j.issn.1009-6663.2014.011.036[6] Movsas B, Scot C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancerpatients receiving chemotherapy and hyperfractionated radiation:radiationtherapy oncology group trial 98-01[J]. J Clin Oncol, 2005, 23(10):2145-2154. DOI:10.1200/JCO. 2005.07.167. [7] Senzer N. A phase Ⅲ randomized evaluation of amifostine in stage ⅢA/ⅢB non-small celllung cancer patients receiving concurrent carboplatin, paclitaxel, and radiation therapy followed by gemcitabine and cisplatin intensification:preliminary findings[J].Semin Oncol, 2002, 29(6 suppl 19):S38-41. DOI:10.1053/sonc.2002.37361. [8] Koukourakis MI. Amifostine:is there evidence of tumor protection?[J]. Semin Oncol, 2003, 30(6 Suppl 18):S18-30. DOI:10.1053/j.seminoncol.2003.11.014. [9] Shuster JJ. Review:Cochrane handbook forsystematic reviews for interventions, Version 5.1.0, published 3/2011. Julian P.T. Higgins and Sally Green, Editors[J]. Res Syn Meth, 2011, 2(2):126-130. DOI:10.1002/jrsm.38. [10] DerSimonian R, Laird N. Meta-analysis in clinical trials[J]. Control Clin Trials, 1986, 7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [11] Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses[J]. BMJ, 2003, 327(7414):557-560. DOI:10.1136/bmj.327.7414.557. [12] Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test[J]. BMJ, 1997, 315(7109):629-634. DOI:10.1136/bmj.315.7109.629. [13] 林展, 严浩林, 朱海生, 等.阿米福汀在局部晚期非小细胞肺癌放疗中的作用研究[J].中国全科医学, 2013, 16(4):1247-1249. DOI:10.3969/j.issn.1007-9572. 2013.04.053.
Lin Z, Yan HL, Zhu HS, et al. Short-term effects of amifostine on locally advanced NSCLC patients treated with radiotherapy[J]. Chin Gen Pract, 2013, 16(4):1247-1249. doi: 10.3969/j.issn.1007-9572.2013.04.053[14] 李永强, 周翔, 张霆.阿米福汀对肺癌放疗中不良反应的影响[J].现代肿瘤医学, 2010, 18(4):708-711. DOI:10.3969/j.issn.1672-4992.2010.04.27.
Li YQ, Zhou X, Zhang T. The effect of amifostine on toxicity of radiotherapy in lung cancer treatment[J]. Mod Oncol, 2010, 18(4):708-711. doi: 10.3969/j.issn.1672-4992.2010.04.27[15] 翁向群, 庄聪文, 程先进, 等.氨磷汀配合紫杉醇和顺铂同步放化疗治疗局部晚期非小细胞肺癌的临床分析[J].临床军医杂志, 2007, 35(6):817-819. DOI:10.3969/j.issn.1671-3826. 2007. 06. 004.
Weng XQ, Zhuang CW, Cheng XJ, et al. Clinical study on efficacy of compound amifostine with radiochemotherapy in treatment of local advanced NSCLC[J]. Clin J Med Office, 2007, 35(6):817-819. doi: 10.3969/j.issn.1671-3826.2007.06.004[16] Komaki R, Lee JS, Milas L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer:report of a randomized comparative trial[J]. Int J Radiat Oncol Biol Phys, 2004, 58(5):1369-1377. DOI:10.1016/j.ijrobp.2003.10.005. [17] Leong SS, Tan EH, Fong KW, et al. Randomized double-blind trial of combined modality treatment with or without amifostine in unresectable stage Ⅲ non-small-cell lung cancer[J]. J Clin Oncol, 2003, 21(9):1767-1774. DOI:10.1200/JCO.2003.11.005. [18] Antonadou D, Throuvalas N, Petridis A, et al. Effect of amifostine on toxicities associated with radiochemotherapy in patients with locally advanced non-small-cell lung cancer[J]. Int J Radiat Oncol Biol Phys, 2003, 57(2):402-408. DOI:10.1016/S0360-3016(03)00590-X. [19] Antonadou D, Coliarakis N, Synodinou M, et al. Randomized phase Ⅲ trial of radiation treatment ±amifostine in patients with advanced-stage lung cancer[J]. Int J Radiat Oncol Biol Phys, 2001, 51(4):915-922. DOI:10.1016/S0360-3016(01)01713-8. [20] Koukourakis MI, Kyrias G, Kakolyris S, et al. Subcutaneous administration of amifostine during fractionated radiotherapy:a randomized phase Ⅱ study[J]. J Clin Oncol, 2000, 18(11):2226-2233. DOI:10.1200/JCO.2000.18.11.2226. [21] Ricciuti B, Brambilla M, Metro G, et al. Targeting NTRK fusion in non-small cell lung cancer:rationale and clinical evidence[J]. Med Oncol, 2017, 34(6):105. DOI:10.1007/s12032-017-0967-5. [22] Antonadou D. Radiotherapy or chemotherapy followed by radiotherapy with or without amifostine in locally advanced lung cancer[J]. Semin Radiat Oncol, 2002, 12(1 suppl 1):S50-58. DOI:10.1053/srao.2002.31374. [23] Koukourakis MI. Amifostine in clinical oncology:current use and future applications[J]. Anticancer Drugs, 2002, 13(3):181-209. DOI:10.1097/00001813-200203000-00001. -






 下载:
下载: